Abstract
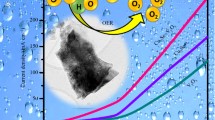
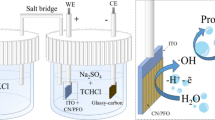
Water electrolysis is a propitious strategy to overcome the exceeding energy crisis by producing renewable and green hydrogen fuel. However, the practical application of this process is limited due to the inadequacy of earth-rich, economical, and efficient electrocatalysts for carrying out kinetically more sluggish oxygen evolution reactions (OER). In the present research, a simple sol–gel method was employed to produce Co3O4/Pr2O3 nanocomposite material, which provides exceptional electrical conductivity and lesser charge transfer resistance of mixed-valence cations. The fabricated nanomaterials were analyzed using various scientific techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HR-TEM), and energy dispersive X-ray spectroscopy (EDX) to determine their crystal structure, morphology, elemental composition, and oxidation states. To investigate the water oxidation capability and steadiness of the modified Co3O4/Pr2O3 electrode material in alkaline conditions, cyclic voltammetry (CV), linear sweep voltammetry (LSV), and constant current chronoamperometry (CA) were utilized. These outcomes revealed that the resultant nanocomposite exhibits a minimal overpotential around 257 mV and a lower Tafel slope around 78 mVdec−1 at a benchmark current density of 10 mAcm−2. In addition, the alkaline solution reliability of the electrocatalysts was examined and confirmed to be steady for 24 h via chronoamperometry. The extraordinary electrocatalytic achievement of Co3O4/Pr2O3 is ascribed to its structural synergistic effect, which encourages the oxygen evolution activity.







Similar content being viewed by others
References
M.H. Jameel, S. Saleem, M. Hashim, M.S. Roslan, H.H.N. Somaily, M.M. Hessin, Z.M. El-Bahy, M.G.B. Ashiq, M.Q. Hamzah, A.H. Jabbar, A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications. Nanotechnol. Rev. 10(1), 1484–1492 (2021)
S. Saleem, M. Irfan, M.Y. Naz, S. Shukrullah, M.A. Munir, M. Ayyaz, A.S. Alwadie, S. Legutko, J. Petrů, S. Rahman, Investigating the impact of Cu2+ doping on the morphological, structural, optical, and electrical properties of CoFe2O4 nanoparticles for use in electrical devices. Materials 15(10), 3502 (2022)
N. Abbas, J.-M. Zhang, S. Nazir, M.T. Ahsan, S. Saleem, U. Ali, N. Akhtar, M. Ikram, R. Liaqat, A comparative study of structural, vibrational mode, optical and electrical properties of pure nickel selenide (NiSe) and Ce-doped NiSe nanoparticles for electronic device applications. Physica B 649, 414471 (2023)
K. Mazloomi, C. Gomes, Hydrogen as an energy carrier: prospects and challenges. Renew. Sustain. Energy Rev. 16(5), 3024–3033 (2012)
L.P. Bicelli, Hydrogen: a clean energy source. Int. J. Hydrogen Energy 11(9), 555–562 (1986)
A. Midilli, M. Ay, I. Dincer, M.A. Rosen, On hydrogen and hydrogen energy strategies: I: current status and needs. Renew. Sustain. Energy Rev. 9(3), 255–271 (2005)
J.M. Petersen, F.U. Zielinski, T. Pape, R. Seifert, C. Moraru, R. Amann, S. Hourdez, P.R. Girguis, S.D. Wankel, V. Barbe, Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476(7359), 176–180 (2011)
K.V. Rao, C. Sunandana, Co3O4 nanoparticles by chemical combustion: effect of fuel to oxidizer ratio on structure, microstructure and EPR. Solid State Commun. 148(1–2), 32–37 (2008)
F. Zhou, Y. Zhou, G.-G. Liu, C.-T. Wang, J. Wang, Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Met. 40, 3375–3405 (2021)
P. Zhou, I.A. Navid, Y. Ma, Y. Xiao, P. Wang, Z. Ye, B. Zhou, K. Sun, Z. Mi, Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 613(7942), 66–70 (2023)
H. Fan, J. Jia, D. Wang, J. Fan, J. Wu, J. Zhao, X. Cui, High-valence Zr-incorporated nickel phosphide boosting reaction kinetics for highly efficient and robust overall water splitting. Chem. Eng. J. 455, 140908 (2023)
F. Zeng, C. Mebrahtu, L. Liao, A.K. Beine, R. Palkovits, Stability and deactivation of OER electrocatalysts: a review. J. Energy Chem. 69, 301–329 (2022)
Y. Lee, J. Suntivich, K.J. May, E.E. Perry, Y. Shao-Horn, Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 3(3), 399–404 (2012)
K.A. Stoerzinger, L. Qiao, M.D. Biegalski, Y. Shao-Horn, Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 5(10), 1636–1641 (2014)
Z. Ma, Y. Zhang, S. Liu, W. Xu, L. Wu, Y.-C. Hsieh, P. Liu, Y. Zhu, K. Sasaki, J.N. Renner, Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@ IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 819, 296–305 (2018)
T. Munawar, S. Yasmeen, M. Hasan, K. Mahmood, A. Hussain, A. Ali, M. Arshad, F. Iqbal, Novel tri-phase heterostructured ZnO–Yb2O3–Pr2O3 nanocomposite; structural, optical, photocatalytic and antibacterial studies. Ceram. Int. 46(8), 11101–11114 (2020)
F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet, X. Hu, Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140(25), 7748–7759 (2018)
J. Wang, H. Kong, J. Zhang, Y. Hao, Z. Shao, F. Ciucci, Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 116, 100717 (2021)
B. Han, A. Grimaud, L. Giordano, W.T. Hong, O. Diaz-Morales, L. Yueh-Lin, J. Hwang, N. Charles, K.A. Stoerzinger, W. Yang, Iron-based perovskites for catalyzing oxygen evolution reaction. J. Phys. Chem. C 122(15), 8445–8454 (2018)
G.K. Wertheim, Hyperfine structure of divalent and trivalent Fe 57 in cobalt oxide. Phys. Rev. 124(3), 764 (1961)
A.Q. Mugheri, A. Tahira, U. Aftab, A.L. Bhatti, R. Lal, M.A. Bhatti, G.Z. Memon, A.B. Mallah, M.A. Abassi, A. Nafady, Chemically coupled cobalt oxide nanosheets decorated onto the surface of multiwall carbon nanotubes for favorable oxygen evolution reaction. J. Nanosci. Nanotechnol. 21(4), 2660–2667 (2021)
J. Wu, Z. Ren, S. Du, L. Kong, B. Liu, W. Xi, J. Zhu, H. Fu, A highly active oxygen evolution electrocatalyst: ultrathin CoNi double hydroxide/CoO nanosheets synthesized via interface-directed assembly. Nano Res. 9, 713–725 (2016)
T. Munawar, F. Mukhtar, M.S. Nadeem, S. Manzoor, M.N. Ashiq, M. Riaz, S. Batool, M. Hasan, F. Iqbal, Facile synthesis of rare earth metal dual-doped Pr2O3 nanostructures: enhanced electrochemical water-splitting and antimicrobial properties. Ceram. Int. 48(13), 19150–19165 (2022)
T. Zhang, S. Zhao, C. Zhu, J. Shi, C. Su, J. Yang, M. Wang, J. Li, J. Li, P. Liu, Rational construction of high-active Co3O4 electrocatalysts for oxygen evolution reaction. Nano Res. 16(1), 624–633 (2022)
X. Xu, Y. Chen, W. Zhou, Z. Zhu, C. Su, M. Liu, Z. Shao, A perovskite electrocatalyst for efficient hydrogen evolution reaction. Adv. Mater. Processes 28(30), 6442–6448 (2016)
S. Dimitrovska-Lazova, S. Aleksovska, V. Mirceski, Pecovska-Gjorgjevich, Correlation between composition, electrical and electrochemical properties of LnCo1–xCrxO3 (Ln= Pr, Gd and x = 0, 0.5 and 1) perovskites. J. Solid State Electrochem.Electrochem. 23, 861–870 (2019)
J. Béjar, L. Álvarez-Contreras, J. Ledesma-García, N. Arjona, L. Arriaga, Electrocatalytic evaluation of Co3O4 and NiCo2O4 rosettes-like hierarchical spinel as bifunctional materials for oxygen evolution (OER) and reduction (ORR) reactions in alkaline media. J. Electroanal. Chem. 847, 113190 (2019)
Y. Song, X. Zhao, Z.-H. Liu, Surface selenium doped hollow heterostructure/defects Co-Fe sulfide nanoboxes for enhancing oxygen evolution reaction and supercapacitors. Electrochim. Acta 374, 137962 (2021)
Q. Zhao, Z. Yan, C. Chen, J. Chen, Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 117(15), 10121–10211 (2017)
H. Ogasawara, A. Kotani, R. Potze, G. Sawatzky, B. Thole, Praseodymium 3d-and 4d-core photoemission spectra of Pr2O3. Phys. Rev. B 44(11), 5465 (1991)
A. Mekki, K.A. Ziq, D. Holland, C. McConville, Magnetic properties of praseodymium ions in Na2O–Pr2O3–SiO2 glasses. J. Magn. Magn. Mater. 260(1–2), 60–69 (2003)
E. Tsuji, A. Imanishi, K.-I. Fukui, Y. Nakato, Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution. Electrochim. Acta 56(5), 2009–2016 (2011)
C.C. McCrory, S. Jung, J.C. Peters, T.F. Jaramillo, Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135(45), 16977–16987 (2013)
Y. Duan, N. Dubouis, J. Huang, D.A. Dalla Corte, V. Pimenta, Z.J. Xu, A. Grimaud, Revealing the impact of electrolyte composition for co-based water oxidation catalysts by the study of reaction kinetics parameters. ACS Catal.Catal. 10(7), 4160–4170 (2020)
Acknowledgements
K.F.F express appreciation to the Deanship of Scientific Research at King Khalid University Saudi Arabia for funding through research groups program under grant number R.G.P. 2/580/44.
Author information
Authors and Affiliations
Contributions
All have done equal contribution.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest by any form for this manuscript.
Ethical standards
Yes this article compliance with ethical standards of journal.
Research data policy and data availability statements
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleem, M.K., Jabbour, K., Niaz, N.A. et al. Facile engineering of Co3O4/Pr2O3 nanostructure for boosted oxygen evolution reaction. Appl. Phys. A 129, 833 (2023). https://doi.org/10.1007/s00339-023-07101-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07101-2