Abstract
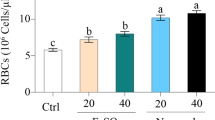
In the current study, bioavailability and biocompatibility of iron oxide–hydroxide (FeOOH) nanospheres (FeNSs) and nanorods (FeNRs) were compared in an in vivo study to investigate the impact of particles shape on efficacy of nano-based iron supplements. FeNSs and FeNRs were fabricated via bio-assisted synthesis reactions using secretory compounds from an edible microalga, Chlorella vulgaris. Resulted FeNSs were ~ 12.7 nm in mean diameter. Mean width of FeNRs were calculated to be 10.8 nm and their mean length was 56.1 nm. Rats were supplemented daily with FeOOH nanostructures and ferrous sulfate. After 1 month, hematologic parameters, serum and organs iron contents, liver function, and organ indexes were evaluated. FeNSs and FeNRs provided significantly increased levels for RBC, liver iron, and spleen iron in contrast to ferrous sulfate. However, just FeNRs were more efficient than ferrous sulfate to elevate the level of Hb (21.9% more than FeSO4) and HTC (22.9% more than FeSO4). It is considered that all examined iron supplements provide an equal level of serum iron. FeOOH nanostructures had no significant effect on toxicological parameters. The only point of concern was increased LDH levels in relation to FeNRs, about 6.4-fold increase was recorded in contrast to untreated group. However, no significant histopathological alterations were detected in FeNRs-treated animals. It can be concluded that nano-based iron supplements are significantly more efficient than iron salts, and the shape of nanostructures can affect their bioavailability. These data can pave the way for future studies toward more efficient and less toxic nano-based iron supplements.






Similar content being viewed by others
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
WHO/UNICEF/UNU. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers: World Health Organization Geneva Switzerland; 2001 [cited 2022]. Available from: https://www.who.int/publications/m/item/iron-children-6to23--archived-iron-deficiency-anaemia-assessment-prevention-and-control
B. De Benoist, M. Cogswell, I. Egli, E. McLean, Worldwide prevalence of anaemia 1993–2005; WHO Global Database of anaemia: WHO; 2008. Available from: https://apps.who.int/iris/handle/10665/43894
J.W. Adamson, Regulation of red blood cell production. Am. J. Med. 101(2), 4S-6S (1996)
S. Donnelly, Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am. J. Kidney Dis. 38(2), 415–425 (2001)
S.E. Graber, S. Krantz, Erythropoietin and the control of red cell production. Annu. Rev. Med. 29(1), 51–66 (1978)
D. Kapur, K. Nath Agarwal, D. Kumari Agarwal, Nutritional anemia and its control. Indian J. Pediatr. 69(7), 607–616 (2002)
N. Berlin, T. Waldmann, S. Weissman, Life span of red blood cell. Physiol. Rev. 39(3), 577–616 (1959)
N. Abbaspour, R. Hurrell, R. Kelishadi, Review on iron and its importance for human health. J. Res. Med. Sci. 19(2), 164–174 (2014)
R. Larocque, M. Casapia, E. Gotuzzo, T.W. Gyorkos, Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am. J. Trop. Med. Hyg. 73(4), 783–789 (2005)
C. WHO, Assessing the iron status of populations: including literature reviews: report of a Joint World Health Organization 2004 [cited 2004]. Available from: https://www.who.int/publications/i/item/9789241596107
L.R. McDowell, Minerals in Animal and Human Nutrition (Elsevier Science BV, USA, 2003)
M.E. Shils, M. Shike, Modern Nutrition in Health and Disease (Lippincott Williams & Wilkins, Philadelphia, PA, 2006)
T. McDonagh, I.C. Macdougall, Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur. J. Heart Fail. 17(3), 248–262 (2015)
A. Ghanbariasad, S.-M. Taghizadeh, P.L. Show, S. Nomanbhay, A. Berenjian, Y. Ghasemi et al., Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae. Bioengineered 10(1), 390–396 (2019)
K. Żebrowska, E. Coy, K. Synoradzki, S. Jurga, P. Torruella, R. Mrówczyński, Facile and controllable growth of β-FeOOH nanostructures on polydopamine spheres. J. Phys. Chem. B. 124(42), 9456–9463 (2020)
S.-M. Taghizadeh, A. Berenjian, M. Zare, A. Ebrahiminezhad, New perspectives on iron-based nanostructures. Processes 8(9), 1128 (2020)
H.M. Naguib, M.A. Ahmed, Z.L. Abo-Shanab, Studying the loading impact of silane grafted Fe2O3 nanoparticles on mechanical characteristics of epoxy matrix. Egypt. J. Pet. 28(1), 27–34 (2019)
P. Kumar, V. Tomar, D. Kumar, R.K. Joshi, M. Nemiwal, Magnetically active iron oxide nanoparticles for catalysis of organic transformations: a review. Tetrahedron 106, 132641 (2022)
S.-I. Ohkoshi, K. Imoto, A. Namai, M. Yoshikiyo, S. Miyashita, H. Qiu et al., Rapid faraday rotation on ε-iron oxide magnetic nanoparticles by visible and terahertz pulsed light. J. Am. Chem. Soc. 141(4), 1775–1780 (2019)
C.H. Le Huy, A.T. Thanh, Study on fabricating epoxy coatings reinforced with iron oxide flakes and nano silica. J. Reinf. Plast. Compos. 42(13–14), 724–740 (2023)
J. Khanam, M.R. Hasan, B. Biswas, S.A. Jahan, N. Sharmin, S. Ahmed et al., Development of ceramic grade red iron oxide pigment from waste iron source. Heliyon 9(1), e12854 (2023)
C. Chen, J. Ge, Y. Gao, L. Chen, J. Cui, J. Zeng et al., Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14(1), e1740 (2022)
E. Christou, J.R. Pearson, A.M. Beltrán, Y. Fernández-Afonso, L. Gutiérrez, J.M. de la Fuente et al., Iron–gold nanoflowers: a promising tool for multimodal imaging and hyperthermia therapy. Pharmaceutics. 14(3), 636 (2022)
D. Stanicki, T. Vangijzegem, I. Ternad, S. Laurent, An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin. Drug Deliv. 19(3), 321–335 (2022)
S. Taghavi, A. Amiri, A. Amin, A. Ehsani, M. Maleki, N. Naderi, Oral iron therapy with polysaccharide-iron complex may be useful in increasing the ferritin level for a short time in patients with dilated cardiomyopathy. Res. Cardiovasc. Med. 6(1), e39816 (2017)
A. Kumari, A.K. Chauhan, Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: a review. J. Food Sci. Technol. 59(9), 3319–3335 (2022)
R. Heidari, S.-M. Taghizadeh, M. Karami-Darehnaranji, E. Mirzaei, A. Berenjian, A. Ebrahiminezhad, Application of FeOOH nano-ellipsoids as a novel nano-based iron supplement: an in vivo study. Biol. Trace Elem. Res. 200(5), 2174–2182 (2022)
M. Karami-Darehnaranji, S.-M. Taghizadeh, E. Mirzaei, R. Heidari, A. Berenjian, A. Ebrahiminezhad, Bio-assisted synthesis of food-grade FeOOH nanoellipsoids as promising iron supplements for food fortification. Appl. Food Biotechnol. 8(1), 71–77 (2020)
A. Albanese, P.S. Tang, W.C. Chan, The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 14(1), 1–16 (2012)
C. Ma, J. Zhang, T. Zhang, H. Sun, J. Wu, J. Shi et al., Comparing the rod-like and spherical BODIPY nanoparticles in cellular imaging. Front. Chem. 7, 765 (2019)
W. Zhang, M. Yu, Z. Xi, D. Nie, Z. Dai, J. Wang et al., Cancer cell membrane-camouflaged nanorods with endoplasmic reticulum targeting for improved antitumor therapy. ACS Appl. Mater. Interfaces 11(50), 46614–46625 (2019)
V. Zavisova, M. Koneracka, A. Gabelova, B. Svitkova, M. Ursinyova, M. Kubovcikova et al., Effect of magnetic nanoparticles coating on cell proliferation and uptake. J. Magn. Magn. Mater. 472, 66–73 (2019)
N.P.D. Sert, V. Hurst, A. Ahluwalia, S. Alam, M.T. Avey, M. Baker et al., The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18(7), e3000410 (2020)
M.M. Ommati, R. Heidari, R.K. Manthari, S. Tikka-Chiranjeevi, R. Niu, Z. Sun et al., Paternal exposure to arsenic resulted in oxidative stress, autophagy, and mitochondrial impairments in the HPG axis of pubertal male offspring. Chemosphere 236, 124325 (2019)
A. Siavashpour, B. Khalvati, N. Azarpira, H. Mohammadi, H. Niknahad, R. Heidari, Poly (ADP-Ribose) polymerase-1 (PARP-1) overactivity plays a pathogenic role in bile acids-induced nephrotoxicity in cholestatic rats. Toxicol. Lett. 330, 144–158 (2020)
C. Luna, M. Ilyn, V. Vega, V.M. Prida, J.N. González, R. Mendoza-Reséndez, Size distribution and frustrated antiferromagnetic coupling effects on the magnetic behavior of ultrafine akaganéite (β-FeOOH) nanoparticles. J. Phys. Chem. C. 118(36), 21128–21139 (2014)
G. Kasparis, A.S. Erdocio, J.M. Tuffnell, N.T.K. Thanh, Synthesis of size-tuneable β-FeOOH nanoellipsoids and a study of their morphological and compositional changes by reduction. CrystEngComm 21(8), 1293–1301 (2019)
Y. Ma, L. Zhang, W. Shi, Y. Niu, B. Zhang, D.J.C.C.L. Su, Facile-fabricated iron oxide nanorods as a catalyst for hydrogenation of nitrobenzene. Chin. Chem. Lett. 30(1), 183–186 (2019)
Y. Piao, J. Kim, H.B. Na, D. Kim, J.S. Baek, M.K. Ko et al., Wrap–bake–peel process for nanostructural transformation from β-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 7(3), 242–247 (2008)
M. Karami-Darehnaranji, S.-M. Taghizadeh, E. Mirzaei, A. Berenjian, A. Ebrahiminezhad, Size tuned synthesis of FeOOH nanorods toward self-assembled nanoarchitectonics. Langmuir 37(1), 115–123 (2020)
A. Ebrahiminezhad, Y. Ghasemi, S. Rasoul-Amini, J. Barar, S. Davaran, Impact of amino-acid coating on the synthesis and characteristics of iron-oxide nanoparticles (IONs). Bull. Korean Chem. Soc. 33(12), 3957–3962 (2012)
A. Ebrahiminezhad, M. Bagheri, S. Taghizadeh, A. Berenjian, Y. Ghasemi, Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv. Nat. Sci. 7, 015018 (2016)
E.P.F. Nhavene, G.F. Andrade, J.A.Q.A. Faria, D.A. Gomes, E.M.B.D. Sousa, Biodegradable polymers grafted onto multifunctional mesoporous silica nanoparticles for gene delivery. ChemEngineering 2(2), 2020024 (2018)
J. Xie, J.Y. Lee, D.I. Wang, Y.P. Ting, Silver nanoplates: from biological to biomimetic synthesis. ACS Nano 1(5), 429–439 (2007)
S.-M. Taghizadeh, N. Lal, A. Ebrahiminezhad, F. Moeini, M. Seifan, Y. Ghasemi et al., Green and economic fabrication of zinc oxide (ZnO) nanorods as a broadband UV blocker and antimicrobial agent. Nanomaterials 10(3), 530 (2020)
J. Yue, X. Jiang, A. Yu, Experimental and theoretical study on the β-FeOOH nanorods: growth and conversion. J. Nanopart. Res. 13(9), 3961–3974 (2011)
N.K. Chaudhari, J.-S. Yu, Size control synthesis of uniform β-FeOOH to high coercive field porous magnetic α-Fe2O3 nanorods. J. Phys. Chem. C. 112(50), 19957–19962 (2008)
B. Tang, G. Wang, L. Zhuo, J. Ge, L. Cui, Facile route to α-FeOOH and α-Fe2O3 nanorods and magnetic property of α-Fe2O3 nanorods. Inorg. Chem. 45(13), 5196–5200 (2006)
K. Chen, X. Chen, D. Xue, Hydrothermal route to crystallization of FeOOH nanorods via FeCl3· 6H2O: effect of Fe3+ concentration on pseudocapacitance of iron-based materials. CrystEngComm 17(9), 1906–1910 (2015)
D. Bakoyannakis, E. Deliyanni, A. Zouboulis, K. Matis, L. Nalbandian, T. Kehagias, Akaganeite and goethite-type nanocrystals: synthesis and characterization. Microporous Mesoporous Mater. 59(1), 35–42 (2003)
M. Xiao, Y. Zhao, S. Li, Facile synthesis of chrysanthemum-like mesoporous α-FeOOH and its adsorptive behavior of antimony from aqueous solution. J. Dispersion Sci. Technol. 41(12), 1812–1820 (2020)
Z. Xu, M. Xie, Y. Ben, J. Shen, F. Qi, Z. Chen, Efficiency and mechanism of atenolol decomposition in Co–FeOOH catalytic ozonation. J. Hazard. Mater. 365, 146–154 (2019)
S.E. Lazic, E. Semenova, D.P. Williams, Determining organ weight toxicity with Bayesian causal models: improving on the analysis of relative organ weights. Sci. Rep. 10(1), 6625 (2020)
S. Zlotkin, P. Arthur, K.Y. Antwi, G. Yeung, Randomized, controlled trial of single versus 3-times-daily ferrous sulfate drops for treatment of anemia. Pediatrics 108(3), 613–616 (2001)
J.D. Cook, M.B. Reddy, Efficacy of weekly compared with daily iron supplementation. Am. J. Clin. Nutr. 62(1), 117–120 (1995)
A.G. Soemantri, Preliminary findings on iron supplementation and learning achievement of rural Indonesian children. Am. J. Clin. Nutr. 50(3), 698–702 (1989)
B. Froessler, C. Cocchiaro, K. Saadat-Gilani, N. Hodyl, G. Dekker, Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: a randomized trial. J. Matern. -Fetal Neonatal Med. 26(7), 654–659 (2013)
Z. Tolkien, L. Stecher, A.P. Mander, D.I. Pereira, J.J. Powell, Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS ONE 10(2), e0117383 (2015)
G. Benoni, L. Cuzzolin, D. Zambreri, M. Donini, P. Del Soldato, I. Caramazza, Gastrointestinal effects of single and repeated doses of ferrous sulphate in rats. Pharmacol. Res. 27(1), 73–80 (1993)
I.M. Zijp, O. Korver, L.B. Tijburg, Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 40(5), 371–398 (2000)
S. Kianpour, A. Ebrahiminezhad, R. Heidari, B. Khalvati, M.-A. Shahbazi, M. Negahdaripour et al., Enterobacter sp. mediated synthesis of biocompatible nanostructured iron-polysaccharide complexes: a nutritional supplement for iron-deficiency anemia. Biol. Trace Elem. Res. 198, 744–755 (2020)
P.K. Saha, L. Saha, Iron nanoparticles and its potential application: a literature review. Indian J. Pharmacol. 53(4), 339–340 (2021)
K.Y. Kim, J.H. Lee, A. Hosseindoust, M.J. Kim, J.Y. Mun, J. Moturi et al., Enhancement of ferrous sulfate absorption using nano-technology in broiler chickens. Livest. Sci. 260, 104869 (2022)
F. Hashem, M. Nasr, Y. Ahmed, Preparation and evaluation of iron oxide nanoparticles for treatment of iron deficiency anemia. Int. J. Pharm. Pharm. Sci. 10(1), 142–146 (2018)
M.A. Elshemy, Iron oxide nanoparticles versus ferrous sulfate in treatment of iron deficiency anemia in rats. Egypt. J. Vet. Sci. 49(2), 103–109 (2018)
R. Augustine, A. Hasan, R. Primavera, R.J. Wilson, A.S. Thakor, B.D. Kevadiya, Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 25, 101692 (2020)
I.M. Adjei, B. Sharma, V. Labhasetwar, Nanoparticles: cellular uptake and cytotoxicity. Adv. Exp. Med. Biol. 811, 73–91 (2014)
P. Foroozandeh, A. Abdul Aziz, Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 13(1), 339 (2018)
S.E. Gratton, P.A. Ropp, P.D. Pohlhaus, J.C. Luft, V.J. Madden, M.E. Napier et al., The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. 105(33), 11613–11618 (2008)
P. Kolhar, A.C. Anselmo, V. Gupta, K. Pant, B. Prabhakarpandian, E. Ruoslahti et al., Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. 110(26), 10753–10758 (2013)
D.M. Teleanu, C. Chircov, A.M. Grumezescu, A. Volceanov, R.I. Teleanu, Impact of nanoparticles on brain health: an up to date overview. J. Clin. Med. 7(12), 490 (2018)
M. Afkhami-Ardakani, A. Shirband, J. Golzade, M. Asadi-Samani, E. Latifi, M. Kheylapour et al., The effect of iron oxide nanoparticles on liver enzymes (ALT, AST and ALP), thyroid hormones (T3 and T4) and TSH in rats. J. Shahrekord Univ. Med. Sci. 14(6), 82–88 (2013)
P. Manna, M. Ghosh, J. Ghosh, J. Das, P.C. Sil, Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: Role of IκBα/NF-κB MAPKs and mitochondrial signal. Nanotoxicology 6(1), 1–21 (2012)
S.M. Smith, M.B. Wunder, D.A. Norris, Y.G. Shellman, A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 6(11), e26908 (2011)
P. Kumar, A. Nagarajan, P.D. Uchil, Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018(6), pdb.prot095497 (2018)
Funding
This study was financially supported by the Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
RH: animal studies and statistical analysis. S-MT: writing the manuscript and graphical artworks. MK-D: preliminary studies and data collection. EM: consultation on study design. AB: writing and editing the manuscript. AE: supervision and grant’s owner.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Iran National Committee approved the study for Ethics in Biomedical Research (Approval ID IR.SUMS.REC.1399.964).
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heidari, R., Taghizadeh, SM., Karami-Darehnaranji, M. et al. Bioavailability and biocompatibility of FeOOH nanostructures as iron supplements: the matter of particle’s shape. Appl. Phys. A 129, 697 (2023). https://doi.org/10.1007/s00339-023-06988-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06988-1