Abstract
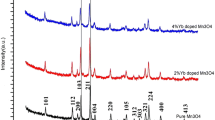
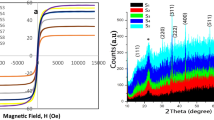
Considering that in the previous work of the group, the physical, electrochemical and catalytic properties of Bi–V–O samples without doping were studied, in this research work we decided to investigate the said properties for samples doped with different ions to compare data and results. Hydrothermal fabrication of some Mx-doped Bi–V–O (M = doped metal) mixed metal oxides was reported in the present study. Several standard techniques, such as energy dispersive X-ray, field emission scanning electron microscopy (FESEM), X-ray powder diffraction, transmission electron microscopy (TEM), ultraviolet–visible (UV–Vis), cyclic voltametry (CV), and vibrating sample magnetometer (VSM), were employed to specify the virtues of the prepared samples. XRD data confirmed that Bi2V2O7 and BiVO4 were the main crystal phases in the mixture of the products. FESEM and TEM images showed that the samples had particle and rod morphologies. The presence of strong spectra in the range of visible light were confirmed using the UV–Vis spectroscopy. The prepared composites' direct band gap energy (Eg) values were among 2 and 5 eV. According to VSM analysis, the results revealed that the samples showed weak magnetic behavior. The highest magnetic property was considered with the intercalating of Fe into the crystal system. Electrochemical properties measured by CV analysis showed that the samples had considerable charge discharge-specific capacitance values. Besides, the samples showed weak oxidation–reduction peaks indicating the possible battery and, or electrocatalyst application. The catalytic performance of the substance was studied for the synthesis of chromene derivatives. A high yield was obtained when ultrasound irradiation was used. In the following, we intend to study the photocatalytic and antibacterial activity of the synthesized samples (pure and doped samples) and compare the results with the components which used in the nanocomposite structure.
Graphical abstract










Similar content being viewed by others
Data availability
All data have been given in the article and supporting information.
References
A. Molinari, R. Argazzi, A. Maldotti, J. Mol. Catal. A Chem. 372, 23–28 (2013)
X.V. Doorslaer, K. Demeestere, P.M. Heynderickx, M. Caussyn, H.V. Langenhove, F. Devlieghere, A. Vermeulen, J. Dewulf, Appl. Catal. B. 138, 333–341 (2013)
L.Z. Pei, S. Wang, Y.K. Xie, H.Y. Yu, Y.H. Guo, J. Alloys Compd. 587, 625–631 (2013)
S. Sorokina, R. Enjalbert, P. Baules, A. Castro, J. Galy, J. Solid State Chem. 144, 379–387 (1999)
S. Beg, N.A.S. Al-Areqi, Philos. Mag. 89, 1279–1294 (2009)
S. Khademinia, M. Behzad, H. Samari Jahromi, RSC Adv. 5, 24313–24318 (2015)
P. Millan, J.M. Rojo, A. Castro, Mater. Res. Bull. 35, 835–845 (2000)
M. Dragomir, M. Valant, Ceram. Int. 39, 5963–5966 (2013)
N. Ramadass, T. Palanisamy, J. Gopalakrishnan, G. Aravamudan, M.V.C. Sastri, Solid State Commun. 17, 545–547 (1975)
L. Wang, X. Shi, Y. Jia, H. Cheng, L. Wang, Q. Wang, Chin. Chem. Lett. 32, 1869–1878 (2021)
O. Monfort, G. Plesch, Environ. Sci. Pol. Res. 25, 19362–19379 (2018)
C.S. Praveen, L. Maschio, M. Rérat, V. Timon, M. Valant, Phys. Rev. B. 96, 165152 (2017)
B. Scola Rodrigues, C. Martins Branco, P. Corio, J.S. Souza, Cryst. Growth Des. 20, 3673–3685 (2020)
B. Khaledi-koureh, L. Kafi-Ahmadi, S. Khademinia, A. Poursattar Marjani. J. Mol. Struct. 1277, 134832 (2023)
A. Poursattar Marjani, F. Asadzadeh, A. Danandeh Asl, Sci. Rep. 12, 22173 (2022)
S. Bikas, A. Poursattar Marjani, S. Bibak, H. SarreshtehdarAslaheh, Sci. Rep. 13, 2564 (2023)
L. Kafi-Ahmadi, S. Khademinia, A. Poursattar Marjani, E. Nozad, Sci. Rep. 12, 19942 (2022)
L. Kafi-Ahmadi, S. Khademinia, A. Poursattar Marjani, P. GozaliBalkanloo, Res. Chem. Intermed. 48, 2973–2986 (2022)
M. Khashaei, L. Kafi-Ahmadi, S. Khademinia, A. Poursattar Marjani, E. Nozad, Sci. Rep. 12, 8585 (2022)
E.A.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A.A. El-Taweel, Heterocycles 26, 903–907 (1987)
G.P. Ellis, The chemistry of heterocyclic compounds, in Chromenes, chromenes, and chromenes. ed. by A. Weissberger, E.C. Taylor (Wiley, New York, 1977)
W.O. Foye, Principi di Chimica Farmaceutica (Padova, Piccin, 1991)
S. Zavar, Arab. J. Chem. 10, S67–S70 (2017)
L. Kafi-Ahmadi, A. Poursattar Marjani, E. Nozad, Appl. Organomet. Chem. 35, e6271 (2021)
L. Zhang, X. Yang, L. Zhang, H. Shu, Y. Jia, L. Qi, Y. Han, R. Wang, J Nanopart Res. 24, 268 (2022)
J. Huo, H. Wei, L. Fu, C. Zhao, C. He, Chin. Chem. Lett. 34, 107261 (2023)
H. Dai, S. Xiong, Y. Zhu, J. Zheng, L. Huang, C. Zhou, J. Deng, X. Zhang, Chin. Chem. Lett. 33, 2590–2594 (2022)
H. Li, L. Wang, Y. Zhu, P. Jiang, X. Huang, Chin. Chem. Lett. 32, 2229–2232 (2021)
Z. Zhang, Z.-W. Hou, H. Chen, P. Li, L. Wang, Green Chem. 25, 3543–3548 (2023)
M.M. Heravi, B. Baghernejad, H.A. Oskooie, J. Chin. Chem. Soc. 55, 659–662 (2008)
T.-S. Jin, J.-C. Xiao, S.-J. Wang, T.-S. Li, Ultrason. Sonochem. 11, 393–397 (2004)
L. Kafi-Ahmadi, S. Khademinia, R. Esmaeili, J. Appl. Chem. 16, 153–166 (2021). https://doi.org/10.22075/CHEM.2021.20994.1872
H. Eshghi, S. Damavandi, G.H. Zohuri, Syn. React. Inorg. Metal-Org. Nano-Metal Chem. 41, 1067–1073 (2011)
B. Liu, L.J. Wu, Y.Q. Zhao, L.Z. Wang, M.Q. Cai, RSC Adv. 6, 92473–92478 (2016)
M. Mousavi, S. Tabatabai Yazdi, G.H. Khorrami, J. Nanostruct. 11, 105–113 (2021)
A. Hakimyfard, M. Samimifar, S. Ostadjoola, S. Khademinia, L. Kafi-Ahmadi, Cryst. Res. Technol. 57, 2200044 (2022)
C. Obuah, M.K. Ainooson, J. Darkwa, RSC Adv. 8, 5362–5371 (2018)
K. Sebayang, D. Aryanto, S. Simbolon, C. Kurniawan, S.F. Hulu, T. Sudiro, M. Ginting, P. Sebayang, I.O.P. Conf, Ser. Mater. Sci. Eng. 309, 012119 (2018)
I. Ali, C. Peng, D. Lin, D. Saroj, I. Naz, Z.M. Khan, M. Sultan, M. Ali, J. Environ. Manage. 234, 273–289 (2019)
B. Xu, S. Yue, Z. Sui, X. Zhang, S. Hou, G. Cao, Y. Yang, Energy Environ. Sci. 4, 2826–2830 (2011)
S. De, C. Purcell, J. Murley, P. Flouda, S. Shah, M. Green, J. Lutkenhaus, Adv. Mater. Interfaces. 5, 1801237 (2018)
R.S. Kate, R.J. Deokate, Mater. Sci. Energy Technol. 3, 830–839 (2020)
Z. Wu, L.P. Sun, T. Xia, L.H. Huo, H. Zhao, A. Rougier, J.C. Grenier, J. Power Sources. 334, 86–93 (2016)
M. Ebrahimi, S. Abdolmohammadi, R. Kia-Kojoori, J. Chin. Chem. Soc. 67, 1895–1902 (2020)
S. Kozuch, J.M.L. Martin, ACS Catal. 2, 2787–2794 (2012)
M. Kargar Karkhah, H. Kefayati, S. Shariati, Appl. Organomet. Chem. 33, e5139 (2019)
S. Maddila, O.A. Abafe, H.N. Bandaru, S.N. Maddila, P. Lavanya, N. Seshadri, S.B. Jonnalagadda, Arab. J. Chem. 12, 3814–3824 (2019)
R. Ballini, G. Bosica, M.L. Conforti, R. Maggi, A. Mazzacanni, P. Righi, G. Sartori, Tetrahedron 57, 1395–1398 (2001)
H. Mehrabi, N. Kamali, J. Iran. Chem. Soc. 9, 599–605 (2012)
M. Khodajoo, S. Sayyahi, S.J. Saghanezhad, Russ. J. Gen. Chem. 86, 1177–1181 (2016)
M.S. Rao, B.S. Chhikara, R. Tiwari, A.N. Shirazi, K. Parang, A. Kumar, Chem. Biol. Interfaces. 2, 362–372 (2012)
K. Gong, H.L. Wang, Z.-L. Fang Liu, Catal. Commun. 9, 650–653 (2008)
S. Balalaie, S. Ramezanpour, M. Bararjanian, J.H. Gross, Synth. Commun. 38, 1078–1089 (2008)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M.D. Farahani, M.H. Beyzavi, H.G. Kruger, Chem. Eng. J. 248, 122–127 (2014)
M. Tajbakhsh, M. Kariminasab, H. Alinezhad, R. Hosseinzadeh, P. Rezaee, M. Tajbakhsh, H. JanatianGazvini, M. Azizi Amiri, J. Iran. Chem. Soc. 12, 1405–1414 (2015)
B. Sadeghi, I. Zarepour, J. Nanostruct. Chem. 5, 305–311 (2015)
B. Karami, M. Farahi, M. Bazrafshan, S. Khodabakhshi, S. Afr, J. Chem. 67, 109–112 (2014)
L. Kheirkhah, M. Mamaghani, N. Mahmoodi, A. Yahyazadeh, S.S. MirnezamiZiabari, J. Chem. Res. 41, 21–24 (2016)
B. Baghernejad, S. Koosha, JACR 14, 19–25 (2020)
Acknowledgements
The authors are grateful to Urmia University for supporting the research.
Author information
Authors and Affiliations
Contributions
MA-M: Data curation; methodology; visualization. LK-A: Conceptualization; project administration; supervision; validation; writing–original draft. APM: Supervision; software; investigation; writing–review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
There is no confict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdi-Mirabadi, M., Kafi-Ahmadi, L. & Poursattar Marjani, A. Physical, electrochemical, and catalytic attributes of Mx–Bi–V–O (Mx = Al, Fe, Co, and Ni) nanocomposites for the fabrication of chromene derivatives. Appl. Phys. A 129, 718 (2023). https://doi.org/10.1007/s00339-023-06967-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06967-6