Abstract
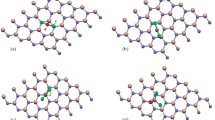
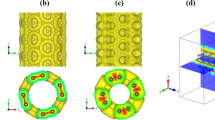
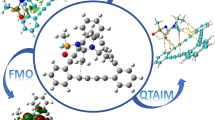
Arsine is the most powerful hemolytic toxin with high flammability and toxicity, which has been used in different areas. Short-term exposure to arsenic might result in a permanent injury or even death. Thus, detection of this gas is of paramount importance. Density functional theory calculations were performed in order to scrutinize the adsorption of XH3 (X = As or P) on a ZnO nano-sheet with a Stone–Wales defect (SWD-ZnONS) and a pristine ZnONS. The interaction of the pure ZnONS with XH3 was predicted to be a physical adsorption. Moreover, the electronic properties of the nano-sheet did not change appreciably. The interaction of AsH3 with the SWD-ZnONS was stronger than that of PH3. The HOMO–LUMO gap of the SWD-ZnONS reduced substantially by approximately − 27.1% when AsH3 was adsorbed, thus raising the electrical conductance significantly. Thus, converting this significant change in electrical conductance into an electronic signal was possible, indicating the possibility of using the SWD-ZnONS as a sensor for detecting AsH3. Furthermore, the adsorption process caused a significant reduction in the work function of the SWD-ZnONS, indicating the possibility of using this nano-sheet as a work function-type sensor to detect AsH3. The computed recovery time for the SWD-ZnONS was 9.5 s for the adsorption of AsH3, which was short. The theoretical findings of this work can provide insights into the practical applications of ZnO nano-structures.









Similar content being viewed by others
Availability of data and materials
All data will be available if required.
Code availability
Not applicable.
References
K. Pande, D. Reep, A. Srivastava, S. Tiwari, J.M. Borrego, S.K. Ghandhi, Device quality polycrystalline gallium arsenide on germanium/molybdenum substrates. J. Electrochem. Soc. 126, 300 (1979)
K.W. Kizer, Toxic inhalations. Emerg. Med. Clin. North Am. 2, 649–666 (1984)
S.F. Rastegar, A.A. Peyghan, N.L. Hadipour, Response of Si-and Al-doped graphenes toward HCN: a computational study. Appl. Surf. Sci. 265, 412–417 (2013)
Y. Zhang, C. Li, H. Ji, X. Yang, M. Yang, D. Jia, X. Zhang, R. Li, J. Wang, Analysis of grinding mechanics and improved predictive force model based on material-removal and plastic-stacking mechanisms. Int. J. Mach. Tools Manuf 122, 81–97 (2017). https://doi.org/10.1016/j.ijmachtools.2017.06.002
S. Gaskin, L. Heath, D. Pisaniello, R. Evans, J.W. Edwards, M. Logan, C. Baxter, Hydrogen sulphide and phosphine interactions with human skin in vitro: Application to hazardous material incident decision making for skin decontamination. Toxicol. Ind. Health 33, 289–296 (2016)
P. Okunieff, S. Swarts, P. Keng, W. Sun, W. Wang, J. Kim, S. Yang, H. Zhang, C. Liu, J.P. Williams, A.K. Huser, L. Zhang, Antioxidants reduce consequences of radiation exposure, in Oxygen Transport to Tissue XXIX. ed. by K.A. Kang, D.K. Harrison, D.F. Bruley (Springer, Boston, 2008), pp.165–178
J.L. Burgess, J. Burgess, Phosphine exposure from a methamphetamine laboratory investigation. J. Toxicol. Clin. Toxicol. 39, 165–168 (2001)
Baker, R. O, & Krieger, R. (2002). Phosphine exposure to applicators and bystanders from rodent burrow treatment with aluminum phosphide. Proceedings of the Vertebrate Pest Conference, 20.
B.D. Banerjee, V. Seth, R.S. Ahmed, Pesticide-induced oxidative stress: Perspective and trends. Rev. Environ. Health 16, 1–40 (2001)
M. Yang, C. Li, Y. Zhang, D. Jia, X. Zhang, Y. Hou, R. Li, J. Wang, Maximum undeformed equivalent chip thickness for ductile-brittle transition of zirconia ceramics under different lubrication conditions. Int. J. Mach. Tools Manuf 122, 55–65 (2017). https://doi.org/10.1016/j.ijmachtools.2017.06.003
Y. Zhang, C. Li, D. Jia, D. Zhang, X. Zhang, Experimental evaluation of the lubrication performance of MoS2/CNT nanofluid for minimal quantity lubrication in Ni-based alloy grinding. Int. J. Mach. Tools Manuf 99, 19–33 (2015). https://doi.org/10.1016/j.ijmachtools.2015.09.003
X. Wang, C. Li, Y. Zhang, Z. Said, S. Debnath, S. Sharma, M. Yang, T. Gao, Influence of texture shape and arrangement on nanofluid minimum quantity lubrication turning. Int. J. Adv. Manuf. Technol. 119, 631–646 (2022). https://doi.org/10.1007/s00170-021-08235-4
X. Cui, C. Li, Y. Zhang, Z. Said, S. Debnath, S. Sharma, H.M. Ali, M. Yang, T. Gao, R. Li, Grindability of titanium alloy using cryogenic nanolubricant minimum quantity lubrication. J. Manuf. Process. 80, 273–286 (2022). https://doi.org/10.1016/j.jmapro.2022.06.003
W. Xu, C. Li, Y. Zhang, H.M. Ali, S. Sharma, R. Li, M. Yang, T. Gao, M. Liu, X. Wang, Electrostatic atomization minimum quantity lubrication machining: from mechanism to application. Int. J. Extreme Manuf. 4, 042003 (2022). https://doi.org/10.1088/2631-7990/ac9652
X. Zhang, C. Li, Y. Zhang, D. Jia, B. Li, Y. Wang, M. Yang, Y. Hou, X. Zhang, Performances of Al2O3/SiC hybrid nanofluids in minimum-quantity lubrication grinding. Int. J. Adv. Manuf. Technol. 86, 3427–3441 (2016). https://doi.org/10.1007/s00170-016-8453-3
Z. Chen, X. He, J. Ge, G. Fan, L. Zhang, A.M. Parvez, G. Wang, Controllable fabrication of nanofibrillated cellulose supported HKUST-1 hierarchically porous membranes for highly efficient removal of formaldehyde in air. Ind Crops Prod 186, 115269 (2022). https://doi.org/10.1016/j.indcrop.2022.115269
J. Li, Z. Liang, Z. Chen, Z. Zhang, H. Liu, Z. Liu, Z. Xu, Engineering unsaturated sulfur site in three-dimension MoS2@ rGO nanohybrids with expanded interlayer spacing and disordered structure for gaseous elemental mercury trap. Chem. Eng. J. 453, 139767 (2023). https://doi.org/10.1016/j.cej.2022.139767
R. Wu, Y. Tan, F. Meng, Y. Zhang, Y.-X. Huang, PVDF/MAF-4 composite membrane for high flux and scaling-resistant membrane distillation. Desalination 540, 116013 (2022). https://doi.org/10.1016/j.desal.2022.116013
S. Lu, J. Guo, S. Liu, B. Yang, M. Liu, L. Yin, W. Zheng, An improved algorithm of drift compensation for olfactory sensors. Appl. Sci. 12, 9529 (2022). https://doi.org/10.3390/app12199529
W. Dang, J. Guo, M. Liu, S. Liu, B. Yang, L. Yin, W. Zheng, A semi-supervised extreme learning machine algorithm based on the new weighted kernel for machine smell. Appl. Sci. 12, 9213 (2022). https://doi.org/10.3390/app12189213
J. Wang, K. Ai, L. Lu, Flame-retardant porous hexagonal boron nitride for safe and effective radioactive iodine capture. J. Mater. Chem. A 7, 16850–16858 (2019). https://doi.org/10.1039/C9TA04489B
M.S. Khan, A. Srivastava, R. Chaurasiya, M.S. Khan, P. Dua, NH3 and PH3 adsorption through single walled ZnS nanotube: first principle insight. Chem. Phys. Lett. 636, 103–109 (2015)
A.R. Víctor, L.D.Q. Pablo, M.Y. Nahuel, General adsorption model for H2S, H2Se, H2Te, NH3, PH3, AsH3 and SbH3 on the V2O5(001) surface including the van der Waals interaction. Chem. Phys. Lett. 720, 58–63 (2019)
M. Noei, A.A. Salari, N. Ahmadaghaei, Z. Bagheri, A.A. Peyghan, DFT study of the dissociative adsorption of HF on an AlN nanotube. C. R. Chim. 16, 985–989 (2013)
A.A. Peyghan, M.T. Baei, M. Moghimi, S. Hashemian, Phenol adsorption study on pristine, Ga-, and In-doped (4, 4) armchair single-walled boron nitride nanotubes. Comput. Theor. Chem. 997, 63–69 (2012)
S. Park, Y.W. Jung, G.M. Ko, D.Y. Jeong, C. Lee, Enhanced NO2 gas sensing performance of the In2O3-decorated SnO2 nanowire sensor. Appl. Phys. A 127, 898 (2021)
S. Park, G.-J. Sun, H. Kheel, T. Ko, H.W. Kim, C. Lee, Light-activated NO2 gas sensing of the networked CuO-decorated ZnS nanowire gas sensor. Appl. Phys. A 122, 504 (2016)
A.A. Peyghan, M. Noei, Fluorination of BC3 nanotubes: DFT studies. J. Mol. Model. 19, 3941–3946 (2013)
A.A. Peyghan, M. Noei, Z. Bagheri, Functionalization of the pristine and stone-wales defected BC3 graphenes with pyrene. J. Mol. Model. 20, 2539 (2014)
J. Kim, M. Ishihara, Y. Koga, K. Tsugawa, M. Hasegawa, S. Iijima, Low-temperature synthesis of large-area graphene-based transparent conductive films using surface wave plasma chemical vapor deposition. Appl. Phys. Lett. 98, 091502 (2011)
L. Wen, F. Li, H.-M. Cheng, Carbon nanotubes and graphene for flexible electrochemical energy storage: From materials to devices. Adv. Mater. 28, 4306–4337 (2016)
Z. Xu, C. Gao, Graphene fiber: A new trend in carbon fibers. Mater. Today 18, 480–492 (2015)
Y.Y. Yang, Y.D. Gong, C.H. Li, X.L. Wen, J.Y. Sun, Mechanical performance of 316L stainless steel by hybrid directed energy deposition and thermal milling process. J. Mater. Process. Technol. 291, 117023 (2021). https://doi.org/10.1016/j.jmatprotec.2020.117023
H. Li, Y. Zhang, C. Li, Z. Zhou, X. Nie, Y. Chen, H. Cao, B. Liu, N. Zhang, Z. Said, Extreme pressure and antiwear additives for lubricant: academic insights and perspectives. Int. J. Adv. Manuf. Technol. (2022). https://doi.org/10.1007/s00170-021-08614-x
B.C. Wood, S.Y. Bhide, D. Dutta, V.S. Kandagal, A.D. Pathak, S.N. Punnathanam, K.G. Ayappa, S. Narasimhan, Methane and carbon dioxide adsorption on edge-functionalized graphene: A comparative DFT study. J. Chem. Phys. 137, 054702 (2012)
T. Gao, Y. Zhang, C. Li, Y. Wang, Y. Chen, Q. An, S. Zhang, H.N. Li, H. Cao, H.M. Ali, Fiber-reinforced composites in milling and grinding: Machining bottlenecks and advanced strategies. Front. Mech. Eng. 17, 1–35 (2022). https://doi.org/10.1007/s11465-022-0680-8
S. Sahoo, S.K. Barik, A.P.S. Gaur, M. Correa, G. Singh, R.K. Katiyar, V.S. Puli, J. Liriano, R.S. Katiyar, Microwave assisted synthesis of ZnO nano-sheets and their application in UV-detector. ECS J. Solid State Sci. Technol. 1, Q140 (2012)
J. Chauhan, N. Shrivastav, A. Dugaya, D. Pandey, Synthesis and characterization of Ni and Cu doped ZnO. J. Nanomed. Nanotechnol 1, 26–34 (2017)
H. Li, Y. Zhang, C. Li, Z. Zhou, X. Nie, Y. Chen, H. Cao, B. Liu, N. Zhang, Z. Said, Cutting fluid corrosion inhibitors from inorganic to organic: Progress and applications. Korean J. Chem. Eng. (2022). https://doi.org/10.1007/s11814-021-1057-0
Y. Xing, Z. Xi, Z. Xue, X. Zhang, J. Song, R. Wang, J. Xu, Y. Song, S.-L. Zhang, D. Yu, Optical properties of the ZnO nanotubes synthesized via vapor phase growth. Appl. Phys. Lett. 83, 1689–1691 (2003)
J. Antony, X. Chen, J. Morrison, L. Bergman, Y. Qiang, D.E. McCready, M.H. Engelhard, ZnO nanoclusters: Synthesis and photoluminescence. Appl. Phys. Lett. 87, 241917 (2005)
Q. Xiao, S. Huang, J. Zhang, C. Xiao, X. Tan, Sonochemical synthesis of ZnO nanosheet. J. Alloy. Compd. 459, L18–L22 (2008)
X. Wang, C. Li, Y. Zhang, H.M. Ali, S. Sharma, R. Li, M. Yang, Z. Said, X. Liu, Tribology of enhanced turning using biolubricants: A comparative assessment. Tribol. Int. (2022). https://doi.org/10.1016/j.triboint.2022.107766
D. Ju, H. Xu, J. Zhang, J. Guo, B. Cao, Direct hydrothermal growth of ZnO nanosheets on electrode for ethanol sensing. Sens. Actuators, B Chem. 201, 444–451 (2014)
A.H. Mashhadzadeh, M. Fathalian, M.G. Ahangari, M. Shahavi, DFT study of Ni, Cu, Cd and Ag heavy metal atom adsorption onto the surface of the zinc-oxide nanotube and zinc-oxide graphene-like structure. Mater. Chem. Phys. 220, 366–373 (2018)
L. Xue, C. Zhang, T. Shi, S. Liu, H. Zhang, M. Sun, F. Liu, Y. Liu, Y. Wang, X. Gu, Unraveling the improved CO2 adsorption and COOH* formation over Cu-decorated ZnO nanosheets for CO2 reduction toward CO. Chem. Eng. J. 452, 139701 (2023)
I. Ayoub, V. Kumar, R. Abolhassani, R. Sehgal, V. Sharma, R. Sehgal, H.C. Swart, Y.K. Mishra, Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 11, 575–619 (2022)
M.W. Schmidt, K.K. Baldridge, J.A. Boatz, S.T. Elbert, M.S. Gordon, J.H. Jensen, S. Koseki, N. Matsunaga, K.A. Nguyen, S. Su, T.L. Windus, M. Dupuis, J.A. Montgomery, J. Comp. Chem. 14, 1347–1363 (1993)
N. O’Boyle, A. Tenderholt, K. Langner, cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845 (2008)
Y.P. Burk, I.A. Koppel, I. Koppel, I. Leito, O. Travnikova, Critical test of performance of B3LYP functional for prediction of gas-phase acidities and basicities. Chem. Phys. Lett. 323, 482–489 (2000)
A.A. Peyghan, M. Moradi, First-principle study of methanol adsorption on Ni (Pd)-decorated graphene. J. Iran. Chem. Soc. 12, 751–756 (2015)
M. Nayebzadeh, A.A. Peyghan, H. Soleymanabadi, Density functional study on the adsorption and dissociation of nitroamine over the nanosized tube of MgO. Phys. E. 62, 48–54 (2014)
A. Hamed Mashhadzadeh, M. Fathalian, M. Ghorbanzadeh Ahangari, M.H. Shahavi, DFT study of Ni, Cu, Cd and Ag heavy metal atom adsorption onto the surface of the zinc-oxide nanotube and zinc-oxide graphene-like structure. Mater. Chem. Phys. 220, 366–373 (2018)
A. Menazea, N.S. Awwad, H.A. Ibrahium, K.H. Alharbi, M.S. Alqahtani, Titanium doping effect on the sensing performance of ZnO nanosheets toward phosgene gas. Phys. Scr. 97, 055816 (2022)
P. Xu, L. Cui, S. Gao, N. Na, A.G. Ebadi, A theoretical study on sensing properties of in-doped ZnO nanosheet toward acetylene. Mol. Phys. 120, e2002957 (2022)
Y. Fang, D.D. Yang, C.Y. Xiang, M. Shi, H. Zhao, H. Asadi, A density functional study on the formaldehyde recognition by Al-doped ZnO nanosheet. J. Mol. Graph. Model. 99, 107630 (2020)
A.A. Peyghan, H. Soleymanabadi, Adsorption of H2S at Stone-Wales defects of graphene-like BC3: a computational study. Mol. Phys. 112, 2737–2745 (2014)
B. Fellmuth, C. Gaiser, J. Fischer, Determination of the Boltzmann constant—status and prospects. Meas. Sci. Technol. 17, R145 (2006)
M. Ali, N. Amrane, N. Tit, Relevance of defects in ZnO nanotubes for selective adsorption of H2S and CO2 gas molecules: Ab-initio investigation. Results in Physics 16, 102907 (2020)
H. Chen, Y. Qu, J. Ding, H. Fu, Adsorption behavior of graphene-like ZnO monolayer with oxygen vacancy defects for NO2: A DFT study. Superlattices Microstruct. 134, 106223 (2019)
A.A. Peyghan, S.P. Laeen, S.A. Aslanzadeh, M. Moradi, Hydrogen peroxide reduction in the oxygen vacancies of ZnO nanotubes. Thin Solid Films 556, 566–570 (2014)
H.B. Michaelson, The work function of the elements and its periodicity. J. Appl. Phys. 48, 4729–4733 (1977)
R. Garg, N.K. Dutta, N. Roy Choudhury, Work function engineering of graphene. Nanomaterials 4, 267–300 (2014)
M. Samadizadeh, S.F. Rastegar, A.A. Peyghan, The electronic response of nano-sized tube of BeO to CO molecule: A density functional study. Struct. Chem. 26, 809–814 (2015)
A. Bano, J. Krishna, D.K. Pandey, N. Gaur, An ab initio study of sensing applications of MoB 2 monolayer: A potential gas sensor. Phys. Chem. Chem. Phys. 21, 4633–4640 (2019)
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding authors
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Ethics approval
We approved all Ethics.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kadhim, M.M., Abdullaha, S.A., Taban, T.Z. et al. The effect of Stone–Wales defect on the sensitivity of a ZnO monolayer in detection of PH3 and AsH3 gases: a DFT study. Appl. Phys. A 129, 159 (2023). https://doi.org/10.1007/s00339-023-06405-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06405-7