Abstract
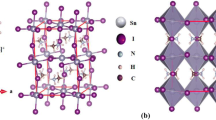
The orthorhombic phase of guanidinium tin halide perovskites C(NH2)3SnX3, X = Cl, Br, I has been studied by quantum chemical method. The lattice parameters are optimized to obtain the minimum energy using the density functional theory with the generalized gradient approximation, GGA-PBE. The Kohn–Sham electronic band structures have been computed; the materials have direct bandgaps of 3.00, 2.47, and 1.78 eV for the C(NH2)3SnCl3, C(NH2)3SnBr3, and C(NH2)3SnI3, respectively, situated at the gamma symmetry points. The projected densities of states are analyzed and the contribution of the p- and s-states of the tin and halogen atoms evaluated. For the GUASnX3 compounds, thermodynamic stability to different decomposition routes has been assessed and standard enthalpies of formation obtained.








Similar content being viewed by others
Data availability
The data collected have been included in the manuscript, and additional data are included in the Supplementary Information.
Code availability
The code used is Quantum Espresso and is freely available.
References
A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131(17), 6050–6051 (2009)
H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J.E. Moser, Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2(1), 1–7 (2012)
NREL (2020) Best Research-Cell Efficiencies. https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200104.pdf. Accessed 08 Apr. 2021
M.M. Lee, J. Teuscher, T. Miyasaka, T.N. Murakami, H.J. Snaith, Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338(6107), 643–647 (2012)
H. Zhou, Q. Chen, G. Li, S. Luo, T.-b Song, H.-S. Duan, Z. Hong, J. You, Y. Liu, Y. Yang, Interface engineering of highly efficient perovskite solar cells. Science 345(6196), 542–546 (2014)
N.J. Jeon, J.H. Noh, W.S. Yang, Y.C. Kim, S. Ryu, J. Seo, S.I. Seok, Compositional engineering of perovskite materials for high-performance solar cells. Nature 517(7535), 476 (2015)
X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai, Y. Lin, H. Wei, X.C. Zeng, J. Huang, Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2(7), 1–9 (2017)
E. Aydin, M. De Bastiani, S. De Wolf, Defect and contact passivation for perovskite solar cells. Adv. Mater. 31(25), 1900428 (2019)
H. Zhang, Y. Wu, C. Shen, E. Li, C. Yan, W. Zhang, H. Tian, L. Han, W.H. Zhu, Efficient and stable chemical passivation on perovskite surface via bidentate anchoring. Adv. Energy Mater. 9(13), 1803573 (2019)
Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin, J. You, Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 13(7), 460–466 (2019)
M. Shahbazi, H. Wang, Progress in research on the stability of organometal perovskite solar cells. Sol. Energy 123, 74–87 (2016)
M. Saliba, T. Matsui, J.-Y. Seo, K. Domanski, J.-P. Correa-Baena, M.K. Nazeeruddin, S.M. Zakeeruddin, W. Tress, A. Abate, A. Hagfeldt, Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9(6), 1989–1997 (2016)
D.P. McMeekin, G. Sadoughi, W. Rehman, G.E. Eperon, M. Saliba, M.T. Hörantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351(6269), 151–155 (2016)
M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S.M. Zakeeruddin, J.-P. Correa-Baena, W.R. Tress, A. Abate, A. Hagfeldt, Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354(6309), 206–209 (2016)
F. Jiang, D. Yang, Y. Jiang, T. Liu, X. Zhao, Y. Ming, B. Luo, F. Qin, J. Fan, H. Han, Chlorine-incorporation-induced formation of the layered phase for antimony-based lead-free perovskite solar cells. J. Am. Chem. Soc. 140(3), 1019–1027 (2018)
X.-G. Zhao, J.-H. Yang, Y. Fu, D. Yang, Q. Xu, L. Yu, S.-H. Wei, L. Zhang, Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 139(7), 2630–2638 (2017)
Z. Shi, J. Guo, Y. Chen, Q. Li, Y. Pan, H. Zhang, Y. Xia, W. Huang, Lead-free organic-inorganic hybrid perovskites for photovoltaic applications: recent advances and perspectives. Adv. Mater. 29(16), 1605005 (2017)
L. Liang, P. Gao, Lead-free hybrid perovskite absorbers for viable application: Can we eat the cake and have it too? Adv. Sci. 5(2), 1700331 (2018)
Z. Xiao, W. Meng, J. Wang, D.B. Mitzi, Y. Yan, Searching for promising new perovskite-based photovoltaic absorbers: the importance of electronic dimensionality. Mater. Horiz. 4(2), 206–216 (2017)
Y.-Y. Sun, J. Shi, J. Lian, W. Gao, M.L. Agiorgousis, P. Zhang, S. Zhang, Discovering lead-free perovskite solar materials with a split-anion approach. Nanoscale 8(12), 6284–6289 (2016)
B. Saparov, F. Hong, J.-P. Sun, H.-S. Duan, W. Meng, S. Cameron, I.G. Hill, Y. Yan, D.B. Mitzi, Thin-film preparation and characterization of Cs3Sb2I9: A lead-free layered perovskite semiconductor. Chem. Mater. 27(16), 5622–5632 (2015)
E.T. McClure, M.R. Ball, W. Windl, P.M. Woodward, Cs2AgBiX6 (X= Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 28(5), 1348–1354 (2016)
A.H. Slavney, T. Hu, A.M. Lindenberg, H.I. Karunadasa, A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 138(7), 2138–2141 (2016)
F. Hong, B. Saparov, W. Meng, Z. Xiao, D.B. Mitzi, Y. Yan, Viability of lead-free perovskites with mixed chalcogen and halogen anions for photovoltaic applications. J. Phys. Chem. C 120(12), 6435–6441 (2016)
C.N. Savory, A. Walsh, D.O. Scanlon, Can Pb-free halide double perovskites support high-efficiency solar cells? ACS Energy Lett. 1(5), 949–955 (2016)
J. Ge, C.R. Grice, Y. Yan, Cu-based quaternary chalcogenide Cu2BaSnS4 thin films acting as hole transport layers in inverted perovskite CH3NH3PbI3 solar cells. J. Mater. Chem. A 5(6), 2920–2928 (2017)
J.T. Dufton, A. Walsh, P.M. Panchmatia, L.M. Peter, D. Colombara, M.S. Islam, Structural and electronic properties of CuSbS2 and CuBiS2: potential absorber materials for thin-film solar cells. Phys. Chem. Chem. Phys. 14(20), 7229–7233 (2012)
Y. Yin, Y. Huang, Y. Wu, G. Chen, W.-J. Yin, S.-H. Wei, X. Gong, Exploring emerging photovoltaic materials beyond perovskite: the case of skutterudite. Chem. Mater. 29(21), 9429–9435 (2017)
W.J. Yin, T. Shi, Y. Yan, Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 26(27), 4653–4658 (2014)
W.-J. Yin, J.-H. Yang, J. Kang, Y. Yan, S.-H. Wei, Halide perovskite materials for solar cells: a theoretical review. J. Mater. Chem. A 3(17), 8926–8942 (2015)
W.-J. Yin, T. Shi, Y. Yan, Superior photovoltaic properties of lead halide perovskites: insights from first-principles theory. J. Phys. Chem. C 119(10), 5253–5264 (2015)
Y. Cho, A.M. Soufiani, J.S. Yun, J. Kim, D.S. Lee, J. Seidel, X. Deng, M.A. Green, S. Huang, A.W. Ho-Baillie, Mixed 3D–2D passivation treatment for mixed-cation lead mixed-halide perovskite solar cells for higher efficiency and better stability. Adv. Energy Mater. 8(20), 1703392 (2018)
P. Chen, Y. Bai, S. Wang, M. Lyu, J.H. Yun, L. Wang, In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 28(17), 1706923 (2018)
S. Tang, Y. Deng, X. Zheng, Y. Bai, Y. Fang, Q. Dong, H. Wei, J. Huang, Composition engineering in doctor-blading of perovskite solar cells. Adv. Energy Mater. 7(18), 1700302 (2017)
D. Chi, S. Huang, M. Zhang, S. Mu, Y. Zhao, Y. Chen, J. You, Composition and interface engineering for efficient and thermally stable pb–sn mixed low-bandgap perovskite solar cells. Adv. Funct. Mater. 28(51), 1804603 (2018)
T. Matsui, T. Yamamoto, T. Nishihara, R. Morisawa, T. Yokoyama, T. Sekiguchi, T. Negami, Compositional engineering for thermally stable, highly efficient perovskite solar cells exceeding 20% power conversion efficiency with 85°C/85% 1000h stability. Adv. Mater. 31(10), 1806823 (2019)
Y. Pei, Y. Liu, F. Li, S. Bai, X. Jian, M. Liu, Unveiling property of hydrolysis-derived DMAPbI3 for perovskite devices: composition engineering, defect mitigation, and stability optimization. Iscience 15, 165–172 (2019)
S. Khalfin, Y. Bekenstein, Advances in lead-free double perovskite nanocrystals, engineering band-gaps and enhancing stability through composition tunability. Nanoscale 11(18), 8665–8679 (2019)
F. Wang, S. Bai, W. Tress, A. Hagfeldt, F. Gao, Defects engineering for high-performance perovskite solar cells. npj Flex. Electron. 2(1), 1–14 (2018)
L. Dimesso, A. Quintilla, Y.-M. Kim, U. Lemmer, W. Jaegermann, Investigation of formamidinium and guanidinium lead tri-iodide powders as precursors for solar cells. Mater. Sci. Eng. B 204, 27–33 (2016)
C.C. Stoumpos, L. Mao, C.D. Malliakas, M.G. Kanatzidis, Structure–band gap relationships in hexagonal polytypes and low-dimensional structures of hybrid tin iodide perovskites. Inorg. Chem. 56(1), 56–73 (2017)
A.D. Jodlowski, C. Roldán-Carmona, G. Grancini, M. Salado, M. Ralaiarisoa, S. Ahmad, N. Koch, L. Camacho, G. De Miguel, M.K. Nazeeruddin, Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2(12), 972–979 (2017)
T. Kishimoto, A. Suzuki, N. Ueoka, T. Oku, Effects of guanidinium addition to CH3NH3PbI3-xClx perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 127(7), 491–497 (2019)
S. Wu, Z. Li, J. Zhang, T. Liu, Z. Zhu, A.K.-Y. Jen, Efficient large guanidinium mixed perovskite solar cells with enhanced photovoltage and low energy losses. Chem. Commun. 55(30), 4315–4318 (2019)
Y. Zhang, J. Chen, X. Lian, M. Qin, J. Li, T.R. Andersen, X. Lu, G. Wu, H. Li, H. Chen, Highly efficient guanidinium-based quasi 2D perovskite solar cells via a two-step post-treatment process. Small Methods 3(11), 1900375 (2019)
R.D. Chavan, D. Prochowicz, M.M. Tavakoli, P. Yadav, C.K. Hong, Surface treatment of perovskite layer with guanidinium iodide leads to enhanced moisture stability and improved efficiency of perovskite solar cells. Adv. Mater. Interfaces 7(1), 2000105 (2020)
E. Vega, M. Mollar, B. Marí, Effect of guanidinium on the optical properties and structure of the methylammonium lead halide perovskite. J. Alloy. Compd. 739, 1059–1064 (2018)
N.K. McKinnon, D.C. Reeves, M.H. Akabas, 5-HT3 receptor ion size selectivity is a property of the transmembrane channel, not the cytoplasmic vestibule portals. J. Gen. Physiol. 138(4), 453–466 (2011)
M. Szafrański, Investigation of phase instabilities in guanidinium halogenoplumbates (II). Thermochim. Acta. 307(2), 177–183 (1997)
G. Giorgi, J.-I. Fujisawa, H. Segawa, K. Yamashita, Organic–inorganic hybrid lead iodide perovskite featuring zero dipole moment guanidinium cations: a theoretical analysis. J. Phys. Chem. C 119(9), 4694–4701 (2015)
G. Giorgi, K. Yamashita, Zero-dipole molecular organic cations in mixed organic–inorganic halide perovskites: possible chemical solution for the reported anomalous hysteresis in the current–voltage curve measurements. Nanotechnology 26(44), 442001 (2015)
N. De Marco, H. Zhou, Q. Chen, P. Sun, Z. Liu, L. Meng, E.-P. Yao, Y. Liu, A. Schiffer, Y. Yang, Guanidinium: a route to enhanced carrier lifetime and open-circuit voltage in hybrid perovskite solar cells. Nano. Lett. 16(2), 1009–1016 (2016)
P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G.L. Chiarotti, M. Cococcioni, I. Dabo, QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21(39), 395502 (2009)
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865 (1996)
C. Paschal, A. Pogrebnoi, T. Pogrebnaya, N. Seriani, Methylammonium tin iodide perovskite: structural, electronic and thermodynamic properties by a DFT study with different exchange-correlation functionals. SN Appl. Sci. 2(4), 1–9 (2020)
P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M.B. Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 29(46), 465901 (2017)
C. Angell, N. Sheppard, A. Yamaguchi, T. Shimanouchi, T. Miyazawa, S. Mizushima, The infra-red spectrum, structure, and normal vibrations of the guanidinium ion. Trans. Faraday Soc. 53, 589–600 (1957)
F. Chiarella, A. Zappettini, F. Licci, I. Borriello, G. Cantele, D. Ninno, A. Cassinese, R. Vaglio, Combined experimental and theoretical investigation of optical, structural, and electronic properties of CH3NH3SnX3 thin films (X= Cl, Br). Phys. Rev. B 77(4), 045129 (2008)
A. Jain, S.P. Ong, G. Hautier, W. Chen, W.D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder, Commentary: the materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1(1), 011002 (2013)
G. Hautier, C. Fischer, V. Ehrlacher, A. Jain, G. Ceder, Data mined ionic substitutions for the discovery of new compounds. Inorg. Chem. 50(2), 656–663 (2011)
M.D. Hanwell, D.E. Curtis, D.C. Lonie, T. Vandermeersch, E. Zurek, G.R. Hutchison, Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 4(1), 1–17 (2012)
K. Momma, F. Izumi, VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41(3), 653–658 (2008)
D.B. Mitzi, Synthesis, crystal structure, and optical and thermal properties of (C4H9NH3)2MI4 (M= Ge, Sn, Pb). Chem. Mater. 8(3), 791–800 (1996)
C.C. Stoumpos, C.D. Malliakas, M.G. Kanatzidis, Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52(15), 9019–9038 (2013)
P. Umari, E. Mosconi, F. De Angelis, Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Sci. Rep. 4, 4467 (2014)
J.P. Perdew, W. Yang, K. Burke, Z. Yang, E.K. Gross, M. Scheffler, G.E. Scuseria, T.M. Henderson, I.Y. Zhang, A. Ruzsinszky, Understanding band gaps of solids in generalized Kohn-Sham theory. Proc. Natl. Acad. Sci. 114(11), 2801–2806 (2017)
Y. Takahashi, R. Obara, Z.-Z. Lin, Y. Takahashi, T. Naito, T. Inabe, S. Ishibashi, K. Terakura, Charge-transport in tin-iodide perovskite CH3NH3SnI3: origin of high conductivity. Dalton Trans. 40(20), 5563–5568 (2011)
I. Borriello, G. Cantele, D. Ninno, Ab initio investigation of hybrid organic-inorganic perovskites based on tin halides. Phys. Rev. B 77(23), 235214 (2008)
J. Even, L. Pedesseau, J.M. Jancu, C. Katan, DFT and k·p modelling of the phase transitions of lead and tin halide perovskites for photovoltaic cells. Phys. Status Solidi (RRL) Rapid Res. Lett. 8(1), 31–35 (2014)
J. Even, L. Pedesseau, J.-M. Jancu, C. Katan, Importance of spin–orbit coupling in hybrid organic/inorganic perovskites for photovoltaic applications. J. Phys. Chem. Lett. 4(17), 2999–3005 (2013)
D.B. Mitzi, C. Feild, Z. Schlesinger, R. Laibowitz, Transport, optical, and magnetic properties of the conducting halide perovskite CH3NH3SnI3. J. Solid State Chem. 114(1), 159–163 (1995)
O. Nazarenko, M.R. Kotyrba, S. Yakunin, M. Wörle, B.M. Benin, G. Rainò, F. Krumeich, M. Kepenekian, J. Even, C. Katan, Guanidinium and mixed cesium-guanidinium tin (ii) bromides: effects of quantum confinement and out-of-plane octahedral tilting. Chem. Mater. 31(6), 2121–2129 (2019)
R. Prasanna, A. Gold-Parker, T. Leijtens, B. Conings, A. Babayigit, H.-G. Boyen, M.F. Toney, M.D. McGehee, Band gap tuning via lattice contraction and octahedral tilting in perovskite materials for photovoltaics. J. Am. Chem. Soc. 139(32), 11117–11124 (2017)
G. Kieslich, S. Sun, A.K. Cheetham, Solid-state principles applied to organic–inorganic perovskites: new tricks for an old dog. Chem. Sci. 5(12), 4712–4715 (2014)
G. Kieslich, S. Sun, A.K. Cheetham, An extended tolerance factor approach for organic–inorganic perovskites. Chem. Sci. 6(6), 3430–3433 (2015)
T. Umebayashi, K. Asai, T. Kondo, A. Nakao, Electronic structures of lead iodide based low-dimensional crystals. Phys. Rev. B 67(15), 155405 (2003)
D. Luo, W. Yang, Z. Wang, A. Sadhanala, Q. Hu, R. Su, R. Shivanna, G.F. Trindade, J.F. Watts, Z. Xu, Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 360(6396), 1442–1446 (2018)
W. Geng, L. Zhang, Y.-N. Zhang, W.-M. Lau, L.-M. Liu, First-principles study of lead iodide perovskite tetragonal and orthorhombic phases for photovoltaics. J. Phys. Chem. C 118(34), 19565–19571 (2014)
C. Quarti, E. Mosconi, J.M. Ball, V. D’Innocenzo, C. Tao, S. Pathak, H.J. Snaith, A. Petrozza, F. De Angelis, Structural and optical properties of methylammonium lead iodide across the tetragonal to cubic phase transition: implications for perovskite solar cells. Energy Environ. Sci. 9(1), 155–163 (2016)
F. Brivio, K.T. Butler, A. Walsh, M. Van Schilfgaarde, Relativistic quasiparticle self-consistent electronic structure of hybrid halide perovskite photovoltaic absorbers. Phys. Rev. B 89(15), 155204 (2014)
V.N. Tuoc, T.D. Huan, Lead-free hybrid organic-inorganic perovskites for solar cell applications. J. Chem. Phys. 152(1), 014104 (2020)
P.-P. Sun, Q.-S. Li, L.-N. Yang, Z.-S. Li, Theoretical insights into a potential lead-free hybrid perovskite: substituting Pb2+ with Ge2+. Nanoscale 8(3), 1503–1512 (2016)
X. Zhao, N.-G. Park, Stability issues on perovskite solar cells. Photonics 2(4), 1139–1151 (2015)
G. Nagabhushana, R. Shivaramaiah, A. Navrotsky, Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites. Proc. Natl. Acad. Sci. 113(28), 7717–7721 (2016)
A. Ciccioli, A. Latini, Thermodynamics and the intrinsic stability of lead halide perovskites CH3NH3PbX3. J. Phys. Chem. Lett. 9(13), 3756–3765 (2018)
B. Brunetti, C. Cavallo, A. Ciccioli, G. Gigli, A. Latini, On the thermal and thermodynamic (in) stability of methylammonium lead halide perovskites. Sci. Rep. 6, 31896 (2016)
I. Ivanov, A. Steparuk, M. Bolyachkina, D. Tsvetkov, A. Safronov, A.Y. Zuev, Thermodynamics of formation of hybrid perovskite-type methylammonium lead halides. J. Chem. Thermodyn. 116, 253–258 (2018)
L. Gurvich, V. Yungman, G. Bergman, I. Veitz, A. Gusarov, V. Iorish, V.Y. Leonidov, V. Medvedev, G. Belov, N. Aristova, Thermodynamic Properties of Individual Substances. Ivtanthermo for Windows Database on Thermodynamic Properties of Individual Substances and Thermodynamic Modeling Software, 3rd edn. (Glushko Thermocenter of RAS, Moscow, 1992).
Y.N. Matyushin, T. Kon’kova, K. Titova, V.Y. Rosolovskii, Y.A. Lebedev, Enthalpy of formation of guanidinium nitrate, perchlorate, and chloride. Bull. Acad. Sci. USSR Div. Chem. Sci. 34(4), 713–716 (1985)
Y. Marcus, The guanidinium ion. J. Chem. Thermodyn. 48, 70–74 (2012)
Acknowledgment
The authors thank the African Development Bank (AfDB) for funding this work.
Funding
The study was funded by the African Development Bank.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paschal, C., Pogrebnoi, A. & Pogrebnaya, T. Guanidinium tin halide perovskites: structural, electronic, and thermodynamic properties by quantum chemical study. Appl. Phys. A 127, 355 (2021). https://doi.org/10.1007/s00339-021-04504-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04504-x