Abstract
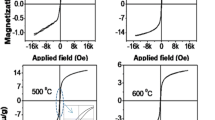
Ferritin is an iron storage protein found in living organisms. It is an antiferromagnetic nanoparticle system consisting of an inorganic core surrounded by a protein shell. Ferritin is characterized by X-ray diffractometer, transmission electron microscope, atomic absorption spectrometer and thermogravimetric analyzer. We find that the ferritin core is poorly crystalline, 8 nm in size and consists of 10 wt% iron. It is believed that cores of ferritin consist of single-phase inorganic mineral ferrihydrite. Recently, we have shown that ferrihydrite decomposes directly to \(\alpha\)-\(\hbox {Fe}_{{2}}\hbox {O}_{3}\) on heating in air at 440 \(^{\circ }\)C. In the present work, we show that ferritin cores gradually decompose to a mixture of \(\gamma\)-\(\hbox {Fe}_{{2}}\hbox {O}_{{3}}\) and \(\alpha\)-\(\hbox {Fe}_{{2}}\hbox {O}_{{3}}\) on heating in air. This mixture finally stabilizes to \(\alpha\)-\(\hbox {Fe}_{{2}}\hbox {O}_{{3}}\) on further heating. The magnetic behaviour of final sample is also studied. This work confirms that the ferritin cores contain more than one phase.







Similar content being viewed by others
References
I. S. Jacobs, C. P. Bean, in Magnetism, Vol. III edited by G. T. Rado, H. Suhl (Academic Press Inc., New York, 1963), p. 271
L. Néel, in Low Temperature Physics, ed. by C. Dewitt, B. Dreyfus, P.D. de Gennes (Gordan and Beach, New York, 1962), p. 413
R.W. Chantrell, K. O’Grady, in Applied Magnetism, ed. by R. Gerber, C.D. Wright, G. Asti (Kluwer Academic Publishers, Amsterdam, 1994), p. 113
R.E. Rosensweig, Ferrohydrodynamics (Cambridge University Press, Cambridge, 1985)
A.S. Edelstein, R.C. Cammarata (eds.), Nanomaterials: Synthesis, Properties and Applications (Taylor & Francis, New York, 1996)
J.L. Jambor, J.E. Dutrizac, Chem. Rev. 98, 2549 (1998)
U. Schwertmann, R.M. Cornell, Iron Oxides in the Laboratory: Preparation and Characterization (Wiley-VCH, Berlin, 2000)
F.M. Michel, L. Ehm, S.M. Antao, P.L. Lee, P.J. Chupas, G. Liu, D.R. Strongin, M.A.A. Schoonen, B.L. Phillips, J.B. Parise, Science 316, 1726 (2007)
M.S. Seehra, V.S. Babu, A. Manivannan, J.W. Lynn, Phys. Rev. B 61, 3513 (2000)
M.S. Seehra, A. Punnoose, Phys. Rev. B 64, 132410 (2001)
A. Punnoose, T. Phanthavady, M.S. Seehra, N. Shah, G.P. Huffman, Phys. Rev. B 69, 54425 (2004)
C. Rani, S.D. Tiwari, J. Magn. Magn. Mater. 385, 272 (2015)
C. Rani, S.D. Tiwari, Phys. B 513, 58 (2017)
D.A. Balaev et al., J. Exp. Theor. Phys. Lett. 98, 139 (2013)
D.A. Balaev et al., J. Exp. Theor. Phys. 119, 479 (2014)
D.A. Balaev et al., Phys. Solid State 58, 1782 (2016)
D.A. Balaev et al., J. Magn. Magn. Mater. 410, 171 (2016)
C. Rani, S.D. Tiwari, Appl. Phys. A 123, 532 (2017)
C. Rani, Ph. D. Thesis, Thapar Institute of Engineering & Technology, Patiala (2018)
J.M. Cowley, D.E. Janney, R.C. Gerkin, P.R. Buseck, J. Struct. Biol. 131, 210 (2000)
S.A. Makhlouf, F.T. Parker, A.E. Berkowitz, Phys. Rev. B 55, R14717 (1997)
N.J.O. Silva et al., Phys. Rev. B 79, 104405 (2009)
N.J.O. Silva et al., Phys. Rev. B 84, 104427 (2011)
S.H. Kilcoyne, R. Cywinski, J. Magn. Magn. Mater. 140–144, 1466 (1995)
N. Kaur, S.D. Tiwari, J. Phys. Chem. Solids 123, 279 (2018)
P.M. Harrison, F.A. Fischbach, T.G. Hoy, G.H. Haggisi, Nature 216, 1188 (1967)
K.M. Towe, W.F. Bradley, J. Colloid Interface Sci. 24, 384 (1967)
S.M. Gorun, G.C. Papaefthymiou, R.B. Frankel, S.J. Lippard, J. Am. Chem. Soc. 109, 3337 (1987)
J.H. Jung, T.W. Eom, Y.P. Lee, J.Y. Rhee, E.H. Choi, J. Magn. Magn. Mater. 323, 3077 (2011)
N.D. Chasteen, P.M. Harrison, J. Struct. Biol. 126, 182 (1999)
F. Brem, G. Stamm, A.M. Hirt, J. Appl. Phys. 99, 123906 (2006)
N. Gálvez, B. Fernández, P. Sánchez, R. Cuesta, M. Ceolín, M. Clemente-León, S. Trasobares, M. López-Haro, J.J. Calvino, O. Stéphan, J.M. Domínguez-Vera, J. Am. Chem. Soc. 130, 8062 (2008)
M. Preisinger, M. Krispin, T. Rudolf, S. Horn, D.R. Strongin, Phys. Rev. B 71, 165409 (2005)
M. Krispin, A. Ullrich, S. Horn, J. Nanopart. Res. 14, 669 (2012)
S. Davis, in Colloid Science Principles, Methods and Applications edited by T. Cosgrove (Wiley, New York, 2010), p. 317
I.M. Weiss, C. Muth, R. Drumm, H.O.K. Kirchner, BMC Biophys. 11, 2 (2018)
V. de la Fuente et al., Minerals 8, 505 (2018)
M. Darbandi et al., J. Phys. D: Appl. Phys. 45, 195001 (2012)
T. Swain, G.S. Brahma, J. Elect. Mater. 47, 2817 (2008)
M. Mobin, Sci. Eng. Compos. Mater. 8, 257 (1999)
M. Mobin, A.U. Malik, S. Ahmad, J. Less Common Metals 160, 1 (1990)
M. Brostrom, S. Enestam, R. Backman, K. Makela, Fuel Process. Technol. 105, 142 (2013)
S. Zhou, Y. Wei, B. Li, H. Wang, B. Ma, C. Wang, Sci. Rep. 6, 1 (2016)
T.A. Rafter, Analyst 75, 1485 (1950)
Y. Goto, Jpn. J. Appl. Phys. 3, 739 (1964)
P. Ayyub, M. Multani, M. Barma, V.R. Palkar, R. Vijayaraghavan, J. Phys. C: Solid State Phys. 21, 2229 (1988)
Y. El Mendili, J.F. Bardeau, N. Randrianantoandro, J.M. Greneche, F. Grasset, Sci. Technol. Adv. Mater. 17, 597 (2016)
G. Gnanaprakash, S. Ayyappan, T. Jayakumar, J. Philip, B. Raj, Nanotechnology 17, 5851 (2006)
M. Tadica, M. Panjanb, V. Damnjanovic, I. Milosevic, Appl. Surf. Sci. 320, 183 (2014)
Acknowledgements
Financial support from University Grant Commission, India is thankfully acknowledged [Project Reference No. 39-537/2010(SR)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, N., Tiwari, S.D. Thermal decomposition of ferritin core. Appl. Phys. A 125, 805 (2019). https://doi.org/10.1007/s00339-019-3104-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3104-9