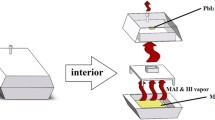
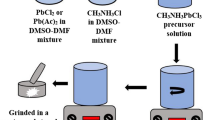
Abstract
Methylammonium lead iodide (CH3NH3PbI3) has attracted tremendous attention in solar cell development due to its direct band gap, ~ 1.50 eV and high optical absorption coefficient, ~ 104–105 cm−1. CH3NH3PbI3 samples were synthesized using a low-cost wet chemical technique and studied their structural, optical, morphological, compositional and thermogravimetric properties. X-ray diffraction pattern of CH3NH3PbI3 revealed a single phase with tetragonal crystal structure; however, the orientation strongly depended on the heating of the sample. Raman spectra exhibited the bending and stretching bonds of Pb–I around 62 and 94 cm−1, respectively, in all samples. The chemical functions of C–H and N–H stretching, C–H and N–H bends, O–H stretch and C–H rock and twist bonds were detected in FTIR spectra. A systematic bond shift revealed higher wavenumber upon increasing the drying temperature demonstrates the growth of stable material. A sharp band edge observed around 1.5 eV confirmed the synthesis of less defective material suitable for high efficiency solar cell development. The content of Pb and I with a desired stoichiometry was determined with EDS measurement. Thermogravimetric analysis demonstrates no weight loss in CH3NH3PbI3 samples up to 220 °C.






Similar content being viewed by others
References
T. Baikie, Y. Fang, J.M. Kadro, M. Schreyer, F. Wei, S.G. Mhaisalkar, M. Graetzeland, T.J. White, Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 1, 5628 (2013)
J.H. Im, C.R. Lee, J.W. Lee, S.W. Park, N.G. Park, 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3, 4088–4093 (2011)
I. Chung, B. Lee, J. He, R.P.H. Chang, M. Kanatzidis, All-solid-state dye-sensitized solar cells with high efficiency. Nature 485, 486–489 (2012)
Green, M.A., Hishikawa, Y., Dunlop, E.D., Levi, D.H. Hohl-Ebinger, J., Yoshita, M., Ho-Baillie, A.W.Y.: Solar cell efficiency tables (version 53). Prog. Photovoltaics Res. Appl. 27(1), 3–12 (2019)
W. Tress, Metal halid perovskites as mixed electronic-ionic conductors: challenges and opportunities from hysteresis to memristivity. J. Phys. Chem. Lett. 8, 3106–3114 (2017)
R. Zhang, J. Fan, X. Zhang, H. Yu, H. Zhang, Y. Mai, T. Xu, J. Wang, H.J. Snaith, Nonlinear optical response of organic−inorganic halide perovskites. Am. Chem. Soc. Photon. 3, 371–377 (2016)
J.Y. Chen, Y.C. Chiu, Y.T. Li, C.C. Chueh, W.C. Chen, Nonvolatile perovskite-based photomemory with a multilevel memory behavior. Adv. Mater. 29, 344–347 (2017)
J.H. Heo, S.H. Im, J.H. Noh, T.N. Mandal, C.S. Lim, J.A. Chang, Y.H. Lee, H.J. Kim, A. Sarkar, M.K. Nazeeruddin, M. Grätzel, S. Seok II, Efficient inorganic−organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat Photon 7, 486–491 (2013)
G. Xing, N. Mathews, S. Sun, S.S. Lim, Y.M. Lam, M. Graẗzel, S. Mhaisalkar, T.C. Sum, Long-range balanced electron and hole-transport lengths in organic−inorganic CH3NH3PbI3. Science 342, 344–347 (2013)
W.S. Yang, B.W. Park, E.H. Jung, N.J. Jeon, Y.C. Kim, D.U. Lee, S.S. Shin, J. Seo, E.K. Kim, J.H. Noh, S.I. Seok, Iodide management in formamidinium–lead–halide-based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017)
A. Halder, D. Choudhary, S. Ghosh, A.S. Subbiah, S.K. Sarkar, Exploring thermochromic behaviour of hydrated hybrid perovskites in solar cells. J. Phys. Chem. Lett. 6, 3180–3184 (2015)
D. Prochowicz, M. Frančkevicius, A.M. Cieślak, S.M. Zakeeruddin, M. Grätzel, J. Lewiński, Mechanosynthesis of the hybrid perovskite CH3NH3PbI3: characterization and the corresponding solar cell efficiency. J. Mater. Chem. A 3, 20772–20777 (2015)
A. Poglitsch, D. Weber, Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 87, 6373–6378 (1987)
S.D. Stranks, G.E. Eperon, G. Grancini, C. Menelaou, M.J.P. Alcocer, T. Leijtens, L.M. Herz, A. Petrozza, H.J. Snaith, Electron–hole diffusion lengths exceeding 1 μm in an organometaltrihalide perovskite absorber. Science 234, 341–344 (2013)
B.J. Foley, D.L. Marlowe, K. Sun, W.A. Saidi, L. Scudiero, M.C. Gupta, J.J. Choi, Temperature dependent energy levels of methylammonium lead iodide perovskite. Appl. Phys. Lett. 106, 243904 (2015)
V. D’Innocenzo, G. Grancini, M.J.P. Alcocer, A.R.S. Kandada, S.D. Stranks, M.M. Lee, G. Lanzani, H.J. Snaith, A. Petrozza, Excitons versus free charges in organo-lead tri-halide perovskites. Nat Commun 5, 3586 (2014)
G.R. Kumar, A.D. Savariraj, S.N. Karthick, S. Selvam, B. Balamuralitharan, H.-J. Kim, K.K. Viswanathan, M. Vijaykumar, K. Prabakar, Phase transition kinetics and surface binding states of methylammonium lead iodide perovskite. Phys. Chem. Chem. Phys. 18, 7284 (2016)
Z. Wang, J. Liu, Z.Q. Xu, Y. Xue, L. Jiang, J. Song, F. Huang, Y. Wang, Y.L. Zhong, Y. Zhang, Y.B. Cheng, Q. Bao, Wavelength-tunable waveguides based on polycrystalline organic-inorganic perovskite microwires. Nanoscale 8, 6258–6264 (2016)
P. Fan, D. Gu, G.X. Liang, J.T. Luo, J.L. Chen, Z.H. Zheng, D.P. Zhang, High-performance perovskite CH3NH3PbI3 thin films for solar cellsprepared by single-source physical vapour deposition. Sci. Rep. 6, 29910–29919 (2016)
P.I. Cowin, R. Lan, C.T.G. Petit, D. Du, K. Xie, H. Wang, S. Tao, Conductivity and redox stability of new perovskite oxides SrFe0.7TM0.2Ti0.1O3 − δ (TM = Mn, Fe Co, Ni, Cu). Solid State Ionics 301, 99–105 (2017)
S. Luo, W.A. Daoud, Crystal structure formation of CH3NH3PbI3 − xClx perovskite. Materials 9, 123 (2016)
X. Guo, C. McCleese, C. Kolodziej, A.C.S. Samia, Y. Zhao, C. Burda, Identification and characterization of the intermediate phase in hybrid organic-inorganic MAPbI3 perovskite. Dalton Trans. 45, 3806 (2016)
B.R. Vincent, K.N. Robertson, T.S. Cameron, O.P. Knop, Alkylammonium lead halide. Part 1. Isolated PbI6 4 − ions in (CH3NH3)4PbI6·2H2O. Can. J. Chem. 65, 1042 (1987)
T. Oku, M. Zushi, Y. Imanishi, A. Suzuki, K. Suzuki, Microstructures and photovoltaic properties of perovskite type CH3NH3PbI3 compound. Appl. Phys. Express 7, 121601 (2014)
R. Gottesman, E. Haltzi, L. Gouda, S. Tirosh, Y. Bouhadana, A. Zaban, E. Mosconi, F. De Angelis, Extremely slow photoconductivity response of CH3NH3PbI3 perovskites suggesting structural changes under working conditions. J. Phys. Chem. Lett. 5, 2662–2669 (2014)
Oku, T.: Crystal structures of CH3NH3PbI3 and related perovskite compounds used for solr cells. In: Solar Cells, New Appeoaches and Reviews, Chapter 3, pp. 77–101. Intechopen
H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, G.V. Gustafsson, M.T. Trinh, S. Jin, X.Y. Zhu, Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 14, 636–642 (2015)
C. Quarti, G. Grancini, E. Mosconi, P. Bruno, J.M. Ball, M.M. Lee, H.J. Snaith, A. Petrozza, F.D. Angelis, The Raman spectrum of the CH3NH3PbI3 hybrid perovskite: interplay of theory and experiment. J. Phys. Chem. Lett. 5, 279–284 (2014)
J. Zhang, Z. Hua, L. Huanga, G. Yuea, J. Liua, X. Lua, Z. Hua, M. Shangb, L. Hanc, Y. Zhu, Bifunctional alkyl chain barriers for efficient perovskite solar cells. Chem. Commun. 51, 7047–7050 (2015)
A. Mishra, Z. Ahmad, F. Touati, R.A. Shakoor, M.K. Nazeeruddin, One-dimensional facile growth of MAPbI3 perovskite micro-rods. RSC Adv. 9, 11589 (2019)
S. Sun, T. Salim, N. Mathews, M. Duchamp, C. Boothroyd, G. Xing, T.C. Sum, Y.M. Lam, The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 7, 399–407 (2014)
A. Dualeh, P. Gao, S. Seok II, M.K. Nazeeruddin, M. Gratzel, Thermal behavior of methylammonium lead–trihalide perovskite photovoltaic light harvesters. J Chem Matter 26, 6160–6164 (2014)
W. Xie, Z. Gao, W.P. Pan, D. Hunter, A. Singh, R. Vaia, Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem. Mater. 13, 2979–2990 (2001)
Acknowledgements
The financial support received from UPE Phase-II and Department of Science and Technology under Solar Energy Research Initiative DST/TM/SERI/FR/124(G) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A., Chaure, N.B. Studies on CH3NH3PbI3 prepared by low-cost wet chemical technique. Appl. Phys. A 125, 767 (2019). https://doi.org/10.1007/s00339-019-3047-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3047-1