Abstract
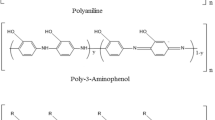
Here, we adopted a donor–acceptor criteria for charge transfer and synthesize the thermally stable copolymers of poly(aniline-co-2-hydroxyaniline) (PA-co-2-HA) by in-situ copolymerization method having different compositions. The co-monomers used in the synthesis were aniline and 2-hydroxyaniline to obtain the (PA-co-2-HA). UV–Vis spectroscopy was used to see the change in bandgap (Eg) between HOMO and LUMO for the electronic transitions. FT-IR analysis has been performed to get functional details of polymers. The electrical conductivity copolymer was recorded by the two-probe method. The conductivity of copolymer depends upon the amount of molar feed in the composition. To probe the surface morphology and roughness profile, atomic force microscopy (AFM) has been applied. The thermal stability of the copolymers (PA-co-2-HA)s has been studied by thermogravimetric analysis (TGA). The particle size of the copolymer varies in the range of 100–500 nm as determined by particle size analyzer. The SEM analysis has been carried out to study the morphological behavior of the copolymer. 1H-NMR spectroscopy was used to study the structural details of the protons present in the copolymer.










Similar content being viewed by others
References
S.H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J.S. Moon, D. Moses, M. Leclerc, K. Lee, A.J. Heeger, Bulk heterojunction solar cells with internal quantum efficiency approaching. Nat. Photonics 3, 297 (2009)
J. Hou, H.-Y. Chen, S. Zhang, R.I. Chen, Y. Yang, Y. Wu, G. Li, Synthesis of a low band gap polymer and its application in highly efficient polymer solar cells. J. Am. Chem. Soc. 131, 15586 (2009)
H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu, G. Li, Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics. 3, 649 (2009)
S. Hellstrom, J. Lars, A. Lindgren, Y Zhou, F. Zhang, O. Inganas, Synthesis and characterization of three small band gap conjugated polymers for solar cell applications. Polym. Chem. 1, 1272 (2010)
J. Roncali, Synthetic principles for bandgap Control in linear π-conjugated systems. Chem. Rev. 97, 173 (1997)
J.H. Burroughes, D.D.C. Bradey, A.R. Brown, R.N. Marks, K. Mackay, R.H. Friend, P.L. Burn, A.B. Holmes, Light-emitting diodes based on conjugated polymers. Nature. 347, 539 (1990)
D.R. Baigent, P.J. Hamer, R.H. Friend, S.C. Moratti, A.B. Holmes, Polymer electroluminescence in the near infra-red. Synth. Met. 71, 2175 (1995)
V.D. Parkar, Energetics of electrode reactions. II. The relationship between redox potentials, ionization potentials, electron affinities, and solvation energies of aromatic hydrocarbons. J.Am. Chem.Soc. 98(1), 98 (1976)
L.E. Lyons, Energy gaps in organic semiconductors derived from electrochemical data. Aust. J.Chem. 33, 1717 (1980)
R.O. Loutfy, Y.C. Cheng, Defect state model and effect of transition metal impurities on metal-free phthalocyanine: electrical and photoconductive properties. J.Chem.Phys. 73, 2911 (1980)
J.P. Lowe, S.A. Kafafi, Effects of chemical substitution on polymer band gaps: transferability of band-edge energies. J.Am.Chem.Soc. 106, 5837 (1984)
Z.G. Soos, G.W. Hayden, Site energies for π-electron models of conjugated polymers. Synth.Met. 28, D543 (1989)
G. Han, Y. Liu, L. Zhang, E. Kan, S. Zhang, J. Tang, W. Tang, MnO2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Scientific Reports 4, 1–7 (2014)
K. Deb, A. Bera, B. Saha, Tuning of electrical and optical properties of polyaniline incorporated functional paper for flexible circuits through oxidative chemical polymerization. RSC Adv. 6, 94795 (2016)
S. Bai, Y. Zhao, J. Sun, Y. Tian, R. Luo, D. Li, A. Chen, Ultrasensitive room temperature NH3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem.Comm. 51, 7524 (2015)
J. Zhang, D. Shan, S. Mu, A promising copolymer of aniline and m-aminophenol: Chemical preparation, novel electric properties and characterization. Polymer 48, 1269 (2007)
P. Saini, V. Choudhary, S. Details, Electrical properties, and electromagnetic interference shielding response of processable copolymers of aniline. J. Mater. Sci. 48, 797 (2013)
H. Yoon, B.M. Jung, H. Lee, Electrical transport in conductive blends of polyaniline in poly(methyl methacrylate). Synth. Met. 63,47, (1994)
S.K. Dhawan, D.C. Trivedi, Poly (o-phenetidine)- a soluble conducting polymer: synthesis, characterization and its uses. Synth. Met. 60, 63 (1993)
P. Saini, R. Jalan, S.K. Dhawan, Synthesis and characterization of processable polyaniline doped with novel dopant NaSIPA. J. Appl. Polym. Sci. 108, 1437 (2008)
A.G. MacDiarmid, J.C. Chiang, A.F. Richter, A.J. Epstein., Polyaniline: a new concept in conducting polymers. Synth. Met. 18, 285–290 (1987)
K. Tzou, R.V. Gregory, A method to prepare soluble polyaniline salt solutions - in situ doping of PANI base with organic dopants in polar solvents. Synth. Met. 53, 365 (1993)
M. Yang, K. Cao, L. Sui, Q. Ying, J. Zhu, A. Waas, E.M. Arruda, J. Kieffer, M.D. Thouless, N.A. Kotov, Dispersions of aramid nanofibers: a new nanoscale building block. ACS Nano. 5(9), 6945 (2011)
X. Jing, Y. Wang, D. Wu, J. Qiang, Sonochemical synthesis of polyaniline nanofibers. Ultrasonics Sonochem. 14, 75 (2007)
R.M. Silverstein, F.X. Webster, Identification of organic compounds, Wiley, Inc,Edition 7th, published. 88 (2005)
V.G. Kulkarni, L.D. Cambell, W.R. Mathew, Thermal stability of polyaniline. Synth. Met. 30, 321 (1989)
P. Kar, N. Pradhan, C. Adhikari, A novel route for the synthesis of processable conducting poly(m-aminophenol),Material Chemistry and Physics, 1, 59 (2008)
T. Gopalaswamy, M. Gopalaswamy, M. Gopichand, Poly meta-aminophenol: chemical synthesis, characterization and AC impedance study. J. Polym. 11, 827043 (2014)
U.S. Waware, A.M.S. Hamouda, M. Rashid, G..J.Summers, The spectral and morphological studies of the conductive polyaniline thin film derivatives by the in situ copolymerization. J. Mater. Sci.: Mater. Electron. 28, 15178 (2017)
H. Mark, in Der feste Körper, ed. by R. Sänger (Hirzel, Leipzig, 1938), pp. 65–104
R. Houwink, Zusammenhang zwischen viscosimetrisch undosmotisch bestimm-ten polymerisationsgraden bei hochpolymeren. J. Prakt. Chem. 157, 15 (1940)
L. Sapna Jadoun, U. Biswal, Riaz, Designed monomer and polymers, vol.21 no.1 75–81 (2018)
L.J. Fetters, J.S. Lindner, J.W. Mays, J. Phys. Chem. Ref. Data, Vol. 23, No. 4 (1994)
Acknowledgements
We acknowledge the Qatar University, Doha, for providing required research fund to carry out the work. We do acknowledge the support for the instrumental analysis of sample by Central Lab Unit (CLU) and Centre for Advance Material (CAM) of the University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Waware, U.S., Hamouda, A.M.S. & Rashid, M. Poly(aniline-co-2-hydroxyaniline): towards the thermal stability and higher solubility of polyaniline. Appl. Phys. A 125, 127 (2019). https://doi.org/10.1007/s00339-019-2418-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2418-y