Abstract
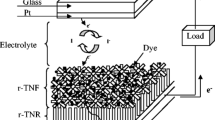
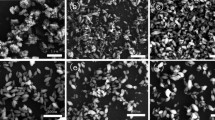
Rutile TiO2 nanorod arrays (TNAs) incorporating with α-alumina (α-Al2O3) thin film have been fabricated on the fluorine-doped tin oxide (FTO) by a modest and flexible doctor-blade technique-based hydrothermal method. The crystallinity of α-Al2O3 on TNAs and morphological control of the photo-anodes were characterized by X-ray diffraction (XRD), UV–Vis spectrophotometry, field emission scanning electron microscopy (FESEM), energy-dispersive spectrometric (EDS) and high-resolution transmission electron microscopy (HRTEM). The growth of α-Al2O3 crystals is influenced by aluminium seed concentrations. The growth mechanism of different morphological TNAs due to the incorporation of α-Al2O3 was discussed in detail. It has also demonstrated to the application of dye sensitized solar cells. Dye-sensitized solar cells (DSSCs) prepared with 3% α-Al2O3 incorporated TNAs shows an improved short-circuit current density of 15.23 mA cm−2, open-circuit photo voltage of 0.68 V, fill factor of 0.63 and a power conversion efficiency of 6.5%.









Similar content being viewed by others
References
S.S. Mao, X. Chen, Selected nanotechnologies for renewable energy applications. Int. J. Energy. Res. 31(6–7), 619–636 (2007)
M. Kitano, M. Matsuoka, M. Ueshima, M. Anpo, Recent developments titanium oxide-based photocatalysts. Appl. Catal. A. Gen. 325, 1–14 (2007)
B. O’Regan, M. Gratzel, Nature. (1991), 353, 737
M. Gratzel, Acc. Chem. Res. (2009), 42, 1788
Y.T. Kim, J. Park, S. Kim, D.W. Park, J. Choi, Electrochim. Acta. 78, 417 (2012)
C.Y. Jiang, X.W. Sun, G.Q. Lo, D.L. Kwong, J.X. Wang, Appl. Phys. Lett. 90, 263501 (2007)
J.B. Baxter, E.S. Aydil, Appl. Phys. Lett. 86, 053114 (2005)
T.S. Senthil, A.Y. Kim, N. Muthukumarasamy, M. Kang, J. Nanopart. Res. 15, 1926 (2013)
T. Ling, J.G. Song, X.Y. Chen, J. Yang, S.Z. Qiao, X.W. Du, J. Alloys. Compd. 546, 307–313 (2013)
J.R. Jennings, A. Ghicov, L.M. Peter, P. Schmuki, A.B. Walker, J. Am. Chem. Soc. 130, 13364–13372 (2008)
S.H. Ko, D. Lee, H.W. Kang, K.H. Nam, J.Y. Yeo, S.J. Hong, Nano. Lett. 11, 666 (2011)
D. Miaoqiang Lv, M. Zheng, L. Ye, J. Sun, W. Xiao, C. Guo, Lin, Nanoscale. 4, 5872–5879 (2012)
J.J. Wu, C.C. Yu, J. Phys. Chem. B 108, 3377–3379 (2004)
Y. Liu, H. Wang, Y. Wang, H. Xu, M. Li, H. Shen, Chem. Commun. 47, 3790–3792 (2011)
X.J. Feng, J. Zhai, L. Jiang, Angew. Chem. 44, 5115–5118 (2005)
S. Fujihara, E. Hosono, K. Kakiuchi, H. Imai, J. Am. Chem. Soc. 126, 7790–7791 (2004)
X.J. Feng, K. Shankar, M. Paulose, C.A. Grimes, Angew. Chem. 48, 8095–8098 (2009)
P. Roy, D. Kim, K. Lee, E. Spiecker, P. Schmuki, Nanoscale. 2, 45–59 (2010)
M. Shanmugam, M.F. Baroughi, D. Galipeau, Thin. Solid. Films. 518, 2678 (2010)
A. Zaban, S.G. Chen, S. Chappel, B.A. Gregg, Chem. Commun. 0(22), 2231–2232 (2000)
S. Wu, H. Han, Q. Tai, J. Zhang, S. Xu, C. Zhou, Y. Yang, H. Hu, B. Chen, X.Z. Zhao, J. Power. Sour. 182, 119 (2008)
M. Grätzel, Nature. 414, 338–344 (2001)
H. Krebs, Fundamentals of Inorganic Crystal Chemistry (McGraw-Hill, London, 1968), p. 242
H. Liu, G. Ning, Z. Gan, Y. Lin, A simple procedure to prepare spherical α-alumina powders. Mater. Res. Bull. 44, 785–788 (2009)
Y. Akila, N. Muthukumarasamy, S. Agilan, D. Velauthapillai, T.S. Senthil, Senthilarasu sundaram, natural dye sensitized TiO2 nanorods assembly of broccoli shape based solar cells. J. Photochem. Photobiol. B 148, 223–231 (2015)
B.D. Cullity, Elements of X-ray Diffraction, 2nd edn. (Addison-Wesley, 1978)
M. Okuya, S. Kaneko, K. Hiroshima, I. Yagi, K. Murakami, J. Eur. Ceram. Soc. 21, 2099 (2001)
S. Rühle, D. Cahen, J. Phys. Chem. B 108, 17946 (2004)
L. Kronik, Y. Shapira, Surf. Sci. Rep. 37, 1 (1999)
E. Palomares, J.N. Clifford, S.A. Haque, T. Lutz, J.R. Durrant, J. Am. Chem. Soc. 125, 475 (2003)
D. Kuang, S. Ito, B. Wenger, C. Klein, J.E. Moser, R. Humphry-Baker, S.M. Zakeeruddin, M. Gratzel, J. Am. Chem. Soc. 128, 4146 (2006)
G.K. Mor, K. Shankar, M. Paulose, O.K. Varghese, C.A. Grimes, Nano. Lett. 6, 215 (2006)
N.N. Bwana, J. Nanopart. Res. 11, 1905 (2009)
M.K. Nazeeruddin, A. Kay, I. Rodicio, R. Humpry-baker, E. Müller, P. Liska, N. Vlachopoulos, M. Grätzel, J. Am. Chem. Soc. 115, 6382 (1993)
H. Alarcón, M. Hedlund, E.M.J. Johansson, H. Rensmo, A. Hagfeldt, J. Phys. Chem. C 111, 13267 (2007)
W. Guo, C. Xu, X. Wang, S. Wang, C. Pan, C. Lin, Z.L. Wang, J. Am. Chem. Soc. 134, 4437–4441 (2012)
B. Liu, S. Eray, Aydil, Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 131, 3985–3990 (2009)
P.P. Sun, X.T. Zhang, X.P. Liu, L.L. Wang, C.H. Wang, J.K. Yang, Y.C. Liu, J. Mater.Chem. 22, 6389–6393 (2012)
M. Zhun, L. Chen, H. Gong, M. Zi, B. Cao, A novel TiO2 nanorod/nanoparticle composite architecture to improve the performance of dye-sensitized solar cells. Ceram. Int. 40, 2337–2342 (2014)
X.T. Zhang, H. Liu, T. Taguchi, Q.B. Meng, O. Sato, A. Fujishima, Slow interfacial charge recombination in solid-state dye-sensitized solar cell using Al2O3-coated nanoporous TiO2 films. Sol. Energy. Mater. Sol. Cells. 81, 197–203 (2004)
K.H. Park, M. Dhayal, Simultaneous growth of rutile TiO2 as 1D/3D nanorod/nanoflower on FTO in one-step process enhances electrochemical response of photoanode in DSSC. Electrochem. Commun. 49, 47–50 (2014)
Acknowledgements
This work was supported by grant no. 34/14/49/2014-BRNS with ATC from the Board of Research in Nuclear Sciences (BRNS) innovation major project proposal of the Department of Atomic Energy (DAE), Government of India.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sriharan, N., Senthil, T.S., Kang, M. et al. Rutile TiO2 nanorod arrays incorporated with α-alumina for high efficiency dye sensitized solar cells. Appl. Phys. A 125, 118 (2019). https://doi.org/10.1007/s00339-019-2407-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2407-1