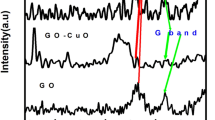
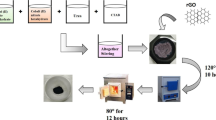
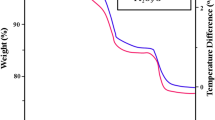
Abstract
In this study, kassite was synthesized by employing a simple, green hydrothermal method. X-ray diffraction, scanning electron microscopy, transmission electron microscopy, cyclic voltammetry, galvanostatic charge/discharge test and electrochemical impedance spectroscopy were carried out to study its crystal phases, morphologies and electrochemical performance. With the extension of reaction time, the crystallinity of the samples became higher and the specific capacitance increased correspondingly. The result shows that kassite has a promising application in electrode material for capacitors. To improve the electrical conductivity of kassite and the accessibility of the surface area, graphene nanosheet (GNS) was introduced to form composites with kassite. The capacitive performance improved with increasing weight percentage of GNS and reached an optimum with the specific capacitance of 129.8 F/g at weight percentage of 10%, then decreased with further increasing GNS, showing a synergistic effect of kassite and the GNS.








Similar content being viewed by others
References
S. Som, A.K. Kunti, V. Kumar, J. Appl. Phys. 115, 193101 (2014)
M. Inoue, A.P. Rodriguez, T. Takagi, J. Biomater. Appl. 24, 657 (2010)
O. Yoko, T. Watanabe, E. Sakai, J. Ceram. Soc. Jpn. 107, 907 (1999)
K. Shimura, H. Yoshida, Energy Environ. Sci. 3, 615 (2010)
C.L. Huang, J.L. Hou, C.L. Pan, J. Alloys Compd. 450, 359 (2008)
Y. Arita, K. Nagarajan, T. Ohashi, T. Matsui, J. Nucl. Mater. 247, 94 (1997)
R. Chen, F. Song, D. Chen, Powder Technol. 194, 252 (2009)
G. Mi, Y. Murakami, D. Shindo, Powder Technol. 105, 162 (1999)
W. Dong, B. Song, G. Zhao, Mater. Res. Bull. 48, 4633 (2013)
P.G. Self, P.R. Buseck, Am. Mineral 76, 283 (1991)
M. Zammarano, M. Franceschi, S. Bellayer, Polymer 46, 9314 (2005)
B. Sels, D. De Vos, M. Buntinx, Nature 400, 855 (1999)
Y. Zhao, Z. Xu, X. Wang, Appl. Surf. Sci. 286, 364 (2013)
J.L. Shumaker, C. Crofcheck, S.A. Tackett, Appl. Catal. B Environ. 82, 120 (2008)
S. Kim, J. Fahel, P. Durand, Eur. J. Inorg. Chem. 2017, 669 (2017)
X. Liu, R. Ma, Y. Bando, Adv. Mater. 24, 2148 (2012)
J.A. Carrasco, H. Prima-Garcia, J. Romero, J. Mater. Chem. C 4, 440 (2016)
N. Mahmood, C. Zhang, H. Yin, J. Mater. Chem. A 2, 15 (2014)
H.L. Guo, X.F. Wang, Q.Y. Qian, ACS Nano 3, 2653 (2009)
S. Chen, J. Zhu, X. Wu, ACS Nano 4, 2822 (2010)
X. Yang, J. Fu, C. Jin, J. Am. Chem. Soc. 132, 14279 (2010)
M.D. Stoller, S. Park, Y. Zhu, Nano Lett 8, 3498 (2008)
M. Zhi, C. Xiang, J. Li, Nanoscale 5, 72 (2013)
G. Wang, L. Zhang, J. Zhang, Chem. Soc. Rev. 41, 797 (2012)
A. Matei, R. Birjega, A. Vlad, Appl. Phys. A 110, 841 (2013)
Z. Gao, J. Wang, Z. Li, Chem. Mater. 23, 3509 (2011)
Z.D. Huang, B. Zhang, R. Liang, Carbon 50, 4239 (2012)
Acknowledgements
This work is supported by National key research and development program (Grant No. 2016YFB0901600), and Major scientific and technological cooperation foundation of Jiangxi Province in China (No. 20161BBH80048).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, W., Zhao, G., Song, B. et al. Facile preparation and electrochemical characterization of kassite-based materials for supercapacitor applications. Appl. Phys. A 123, 753 (2017). https://doi.org/10.1007/s00339-017-1290-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1290-x