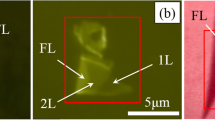
Abstract
The dependence of graphene oxide (GO)-based surface enhanced Raman scattering (SERS) on the pH value of probe molecule was investigated. Water-soluble copper phthalocyanine (TSCuPc) was used as probe molecule and its pH value was adjusted with HCl and NaOH solution. The Raman spectra of TSCuPc with pH equaling 3, 8, and 11 on GO base were tested, respectively. The results show that both Raman enhanced intensity and full width at half maximum (FWHM) of characteristic peaks vary with the pH value of TSCuPc. It is shown that there is no obvious spectral widening of TSCuPc characteristic peaks when TSCuPc is neutral or acidic, and the chemical enhancement intensity of neutral TSCuPc on GO is biggest. In contrast, when TSCuPc is alkaline, the characteristic Raman peaks between 1350 and 1600 cm−1 of TSCuPc on GO are much wider and the intensities of characteristic peaks decrease considerably. The reasons for the pH dependence of GO-based Raman spectra were explored by comparing the wettability of molecule droplet on GO and the absorbance of different pH-adjusted TSCuPc films. It is found that the effect of molecule’s pH value on SERS can be contributed to the differences of concentration and distributions on GO surface for varied pH-treated molecule.







Similar content being viewed by others
References
A. El-Ansary, L.M. Faddah, Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 3(1), 65–76 (2010)
S.D. Hudson, G. Chumanov, Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal. Bioanal. Chem. 394(3), 679–686 (2009)
R.A. Tripp, R.A. Dluhy, Y. Zhao, Novel nanostructure for SERS biosensing. Nano Today 3(3), 31–37 (2008)
Kleinman S.L. PaxtonWF, A.N. Basuray, J.F. Stoddart, R.P. Van Duyne, Surface-enhanced Raman spectroelectrochemistry of TTF-modified self-assembled monolayers. J. Phys. Chem. Lett. 2(10), 1145–1149 (2011)
E. Cortes, P.G. Etchegoin, E.C. Le Ru, A. Fainstein, M.E. Vela, R.C. Salvarezza, Monitoring the electrochemistry of single molecules by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 132(51), 18034–18037 (2010)
M. Sun, Z. Zhang, H. Zheng, X. Hongxing, In-situ plasmon-driven chemical reactions revealed by high vacuum tip-enhanced Raman spectroscopy. Sci. Rep. 2(647), 1–4 (2012)
X.M. Lin, Y. Cui, Y.H. Xu, B. Ren, Z.Q. Tian, Surface-enhanced Raman spectroscopy: substrate-related issues. Anal. Bioanal. Chem. 394(7), 1729–1745 (2009)
B. Ren, G.K. Liu, X.B. Lian, Z.L. Yang, Z.Q. Tian, Raman spectroscopy on transition metals. Anal. Bioanal. Chem. 388(1), 29–45 (2007)
B.N.J. Persson, K. Zhao, Z. Zhang, Chemical contribution to surface-enhanced Raman scattering. Phys. Rev. Lett. 96, 207401 (2006)
S. Huang, X. Ling, L. Liang, Y. Song, W. Fang, J. Zhang et al., Molecular selectivity of graphene-enhanced Raman scattering. Nano Lett. 15, 2892–2901 (2015)
X. Ling, L. Xie, Y. Fang, X. Hua, H. Zhang, J. Kong et al., Can graphene be used as a substrate for Raman enhancement? Nano Lett. 10(2), 553–561 (2010)
X. Ling, W. Juanxia, X. Weigao, J. Zhang, Probing the effect of molecular orientation on the intensity of chemical enhancement using graphene-enhanced Raman spectroscopy. Small 8(9), 1365–1372 (2012)
A. Lerf, H. He, M. Forster, J. Klinowski, Structure of graphite oxide revisited. J. Phys. Chem. B 102(23), 4477–4482 (1998)
D.A. Dikin, S. Stankovich, E.J. Zimney, R.D. Piner, G.H.B. Dommett, G. Exmenenko et al., Preparation and characterization of graphene oxide paper. Nature 448, 457–460 (2007)
Y. Lei, L. Li-jun, C. Hao, Z. Yan-yong, W. Hui-dan et al., Preparation and study of GO decorated with Au nanoparticles as SERS active substrate. J. Light Scatt. 27(4), 320–325 (2015)
H. Yang, H. Hailong, Z. Ni, C.K. Poh, C. Cong, J. Lin et al., Comparison of surface-enhanced Raman scattering on graphene oxide, reduced graphene oxide and graphene surfaces. Carbon 62, 422–429 (2013)
Yu. Xinxin, H. Cai, W. Zhang, X. Li, N. Pan, Y. Luo et al., Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide. Am. Chem. Soc. 5(2), 952–958 (2011)
W. Liang, Yu. Xiaoyun Chen, Y.F. Sa, Y. Wang, W. Lin, Graphene oxide as a substrate for Raman enhancement. Appl. Phys. A 109(1), 81–85 (2012)
L. Zhang, J. Xia, Q. Zhao, L. Liu, Z. Zhang, Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6(4), 537–544 (2010)
X. Min-Min, Y.-X. Yuan, J.-L. Yao, S.-Y. Han, M. Wang, G. Ren-Ao, Adsorption of 2-amino-5-cyanopyridine on a gold surface as probed by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 42, 324–331 (2011)
C.Y. Panicker, H.T. Varghese, L. Ushakumari, Y.S. Mary, J. Sarkar, J. Chowdhury, Concentration and pH dependent SERS spectra of sulfanilic acid sodium salt on colloidal silver particles. J. Raman Spectrosc. 41, 944–951 (2010)
H.A. Becerril, J. Mao, Z. Liu, R.M. Stoltenberg, Z. Bao, Y. Chen, Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2(3), 463–470 (2008)
M. Hirata, T. Gotou, S. Horiuchi, M. Fujiwara, M. Ohba, Thin-film particles of graphene oxide 1:high-yield synthesis and flexibility of the particles. Carbon 42(14), 2929–2937 (2004)
W. Xu, N. Mao, J. Zhang, Graphene: a platform for surface-enhanced Raman spectroscopy. Small 9(8), 1206–1224 (2013)
D. Xiao-feng, G. Rong, C. Pei-zhi, Recent development of contact angle measurement technique. PTCA (Part A Phys. Test) 44(2), 84–89 (2008)
Z. Zhen-guo, Contact angle and its application in surface chemistry research. Chem. Res. Appl. 12(4), 370–374 (2000)
S. Schumann, R.A. Hatton, T.S. Jones, Organic photovoltaic devices based on water-soluble copper phthalocyanine. Phys. Chem. C 115, 4916–4921 (2011)
A.A.M. Farag, Optical absorption studies of copper phthalocyanine thin films. Opt. Laser Technol. 39(4), 728–732 (2007)
H.-Q. Xiang, K. Tanaka, T. Kajiyama, Gas-Sensing properties of dilithium octacyano phthalocyanine Langmuir–Blodgett Films. Langmuir 18(23), 9102–9105 (2002)
Acknowledgements
This work is supported by the Basic Research Cost for National Central University (No. 106112015CDJXY120011) and the visitor fund of Key Laboratory of Optoelectronic Technology and Systems of the Education Ministry of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Sl., Du, Xq., Zeng, C. et al. Effect of pH value of probe molecule on the graphene oxide-based surface enhanced Raman scattering. Appl. Phys. A 123, 421 (2017). https://doi.org/10.1007/s00339-017-1010-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1010-6