Abstract
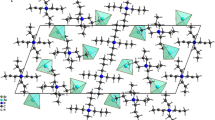
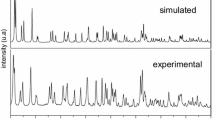
A new organic–inorganic material 3-ammoniumpropyl imidazolium pentabromoantimonate (III) was reported. The title compound was synthesized at room temperature by slow evaporation and then characterized by single-crystal and powder X-ray diffraction, spectroscopic measurements, thermal analysis, and dielectric technique. It crystallizes in the non-centrosymmetric space group P212121 with the following unit cell parameters: a = 8.7120(7), b = 12.6608(1), c = 14.3498(1) Å with Z = 4. The crystal is built up of separated SbBr5 2− polyhedra anions and 3-ammoniumpropyl imidazolium cations. The cohesion of the structure is ensured by network of N–H…Br hydrogen bonds between the 3-ammoniumpropyl imidazolium cations and [SbBr5]2− anions, in which they may be effective in the stabilization of the crystal structure. The Raman and infrared spectra confirm the presence of both cationic [C6H13N3]2+ and anionic [SbBr5]2− entities. Dielectric data were analyzed using complex permittivity ε* and complex electrical modulus M* for the sample at various temperatures. The conductivity follows the Arrhenius low. The Z′ and Zʺ versus frequency plots are well fitted to an equivalent circuit model. The circuits consist of the parallel combination of bulk resistance Rp and constant phase elements CPE. [C6H13N3]SbBr5 crystals undergo one endothermic peak at 333 K. This transition was detected by DSC and by dielectric measurements using the impedance and modulus spectroscopy techniques.















Similar content being viewed by others
References
D.P. Mitzi, Prog, Inorg. Chem. 481(1999)
G.C. Papavassiliou, Prog. Solid State Chem, 25(3–4), 125 (1997)
M. Fra, S. Morimoto, T. Tsutsui, S. Saito, Appl. Phys. Lett. 65(6), 676 (1994)
M. Wojtas, R. Jakubas, Z. Ciunik, W. Medycki, J. Solid State Chem. 177(4–5), 1575 (2004)
G. Bator, Th. Zeegers-Huyskens, R. Jakubas, J. Zaleski, J. Mol. Struct. 570(1), 61 (2001)
B. Kulicka, T. Lis, V. Kinzhybalo, R. Jakubas, A. Piecha, Polyhedron. 29(8), 2014 (2010)
L. Sobczyk, R. Jakubas, J. Zaleski, Pol. J. Chem. 71265 (1997)
H. Ishihara, K. Yamada, T. Okuda, A. Weiss, Bull. Chem. Soc. Jpn 66, 380 (1993)
A. Kallel, J. W. Bats, Acta Crystallogr. C: Cryst. Struct. Commun. C41, 1022 (1985)
M. Bujak, R.J. Angel, J. Solid State Chem 180, 30264 (2007)
G.M. Sheldrick, SORTAV User Guide, Univ. of GÖttingen, Germany (1995)
G.M. Sheldric, SHELXS-97. Program for the solution of crystal structures, Univ. of Göttingen, Germany (1995)
G. M. Sheldric, SHELXS-97. Program for crystal structures determination, Univ. of GÖttingen, Germany (1997)
K. Brandenburg, Diamond Version 2. 0. Impact Gbr Bonn (1998)
L. Pauling, The nature of chemical bond; Itca: Cornell Univ. Press, 260 (1960)
S. Chaabouni, S. Kamoun, J. Jaud, J. Chem Crystallogr. 28, 209 (1998)
J. Zaleski, A. Pietraszko, Acta Crystallogr. B 52/2, 287 (1996)
S. Chaabouni, S. Kamoun, J. Jaud, J. Chem Crystallogr. 28, 209–212 (1998)
H. Khili, N. Chaari, M. Fliyou, A. Koumina, A. Chaabouni, Polyhedron. 36 (2012)
H. Dammak, S. Triki, A. Mlayah, Y. Abid, H. Feki, J. Luminescence. 180–180 (2015)
J. Tarasiewicz, R. akubas, J. Baran, A. Pietraszko, J. Mol Struct. 265–972 (2006)
D. Sajan, J. Binoy, B. Pradeep, K. Venkatakrishnan, V. B. Kartha, I. H. Joe, V. S. Jayakumar, Spectrochim. Acta. A 60–173 (2004)
S. Gunasekaran, S. R. Varadhan, K. Manoharan, Asian J. Phys. 2, 165–172 (1993)
H. Khili, N. Chaari, A. Madani, N.R. Ramond, J. Jaud, S. Chaabouni, Polyhedron. 48–146 (2012)
S. Attia, N. Chaari, S. Chaabouni July, J. Cluster Sci. 26(4), 1343 (2015)
W. Trigui, A. Oueslati, I. Chaabane, F. Hlel, J. Solid State Chem. 227, 10–16 (2015)
J. Tarasiewicz, R. Jakubas, J. Zaleski, J. Baran, J. Mol Struc. 876, 86–101 (2008)
A. Ouasri, H. Jeghnou, A. Rhandou, A. Mazzah, P. Roussel, J. Mol. Struct. 1028, 79 (2012)
K. Azouzi, B. Hamdi, R. Zouari, A. Ben Salah April, Ionics (2016)
A. Ben Rhaiem, F. Hlel, K. Guidara, M. Gargouri, J. Alloy Comp 463, 440–445 (2008)
M. Djemel, M. Abdelhedi, L. Ktari, M. Dammak, J. Mol Struct 1047, 15–21 (2013)
N. Zouari, K. Jaouadi, T. Mhiri, A. Daoud, E. Lebraud, P. Gravereau, J. Mol Struct 842, 81–92 (2007)
A. Ben Chrifa, A. Ben Salah, M. Loukil March, J. Cluster Sci. (2016)
A. Kessentini, M. Belhouchet, Y. Abid, C. Minot, T. Mhiri, Spectrochim Acta. A 122, 476–481 (2014)
R. Elwej, N. Hannachi, I. Chaabane, A. Oueslati, F. Hlel, Inorg Chim Acta 406, 10–19 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taher, M.B., Chaari, N., Bechir, M.B. et al. X-ray diffraction, vibrational properties, and dielectric studies of 3-ammoniumpropyl imidazolium pentabromoantimonate (III). Appl. Phys. A 123, 285 (2017). https://doi.org/10.1007/s00339-017-0895-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0895-4