Abstract
The effect of the acetylacetonate content, a complexing ligand during the one-step sol–gel preparation of TiO2–C hybrid aerogels on their photocatalytic performance for degradation of methylene blue (MB), was investigated. N2 adsorption, XRD, SEM, TEM, Raman and UV–vis spectroscopy were used to characterize the physics-chemical properties of the TiO2–C hybrid aerogels. Results show that complexing ligand can inhibit growth of TiO2 nanoparticles in sol–gel process. Thereby, the porous properties, adsorption equilibrium and kinetics for MB, crystalline size and band gap of the TiO2 in the hybrid aerogels are changed accordingly. The photocatalytic activity of the hybrid aerogels is dominantly determined by adsorption equilibrium and kinetics. Band-gap narrowing, reduced e−–h+ recombination rate and light availability are also responsible for the high photocatalytic activity.
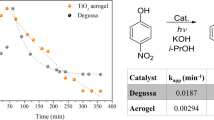
Graphical abstract

A comparison of photocatalytic activity of best sample with P25 for methylene blue degradation








Similar content being viewed by others
Abbreviations
- ρ :
-
Apparent density
- FWHM:
-
Full width at half maximum
- S BET :
-
Surface area by a Brunauer–Emmett–Teller (BET) method
- S ext :
-
External surface area by a t-plot method
- D p :
-
The mean pore size evaluated by BJH (Barret–Joyner–Hallender) pore size distribution curves from desorption branched of the N2 adsorption isotherms
- V mes :
-
Pore volume of mesopores between 2 and 50 nm
- V mic :
-
Pore volume of micropores <2 nm
- Φ :
-
Porosity
- V total :
-
Total pore volume including micropores, mesopores and macropores
- t :
-
Time
- q t :
-
The amount of MB adsorbed at t
- q 2 :
-
The amount of MB adsorbed at equilibrium
- k 2 :
-
The pseudo-second-order rate constant
- \( x_{{{\text{TiO}}_{2} }} \) :
-
Weight percentage of TiO2
- C 0 :
-
The initial concentration of methylene blue
- C :
-
The concentration of methylene blue at time of t
References
J. Matos, J. Laine, J.M. Herrmann, Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B-Environ. 18, 281–291 (1998)
C. Minero, F. Catozzo, E. Pelizzetti, Role of adsorption in photocatalyzed reactions of organic molecules in aqueous titania suspensions. Langmuir 8, 481–486 (1992)
T. Torimoto, S. Ito, S. Kuwabata, Effects of adsorbents used as supports for titanium dioxide loading on photocatalytic degradation of propyzamide. Envrion. Sci. Technol. 30, 1275–1281 (1996)
T. Tsumura, N. Kojitani, I. Izumi, N. Iwashita, Carbon coating of anatase-type TiO2 and photoactivity. J. Mater. Chem. 12, 1391–1396 (2002)
M.L. Chen, C.S. Lim, W.C. Oh, Preparation with different mixing ratios of anatase to activated carbon and their photocatalytic performance. J. Ceram. Process. Res. 8, 119–124 (2007)
R.S. Yuan, R.B. Guan, W.Z. Shen, J.T. Zheng, Photocatalytic degradation of MB by a combination of TiO2 and activated carbon fibers. J. Colloid Interf. Sci. 282, 87–91 (2005)
Y.H. Zhang, Z.R. Tang, X.Z. Fu, Y.J. Xu, TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 4, 7303–7314 (2010)
X. Shao, W.C. Lu, R. Zhang, F. Pan, Enhanced photocatalytic activity of TiO2–C hybrid aerogels for methylene blue degradation. Scientific Reports. 3, 3018 (2013)
H. Irie, Y. Watanabe, K. Hashimoto, Carbon-doped anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem. Lett. 32, 772–773 (2003)
S. Sakthivel, H. Kisch, Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 42, 4908–4911 (2003)
M. Inagaki, F. Kojin, B. Tryba, M. Toyoda, Carbon-coated anatase: the role of the carbon layer for photocatalytic performance. Carbon 43, 1652–1659 (2005)
M. Toyoda, T. Yano, T. Tomoki, Y. Amao, M. Inagaki, Effects of carbon coating on Ti n O2n−1 for decomposition of iminoactadine triacetate in aqueous solution under visible light. J. Adv. Oxid. Technol. 9, 49–52 (2006)
B. Tryba, A.W. Morawski, T. Tsumura, M. Toyoda, M. Inagaki, Hybridization of adsorptivity with photocatalytic activity–carbon-coated anatase. J. Photoch. Photobio. A. 167, 127–135 (2004)
G.L. Puma, A. Bono, D. Krishnaiah, J.G. Collin, Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: a review paper. J. Hazard. Mater. 157, 209–219 (2008)
H. Uchida, S. Itoh, H. Yoneyama, Photocatalytic decomposition of propyzamide using TiO2 supported on activated carbon. Chem. Lett. 22, 1995–1998 (1993)
A.H. El-Sheikh et al., Deposition of anatase on the surface of activated carbon. Surf. Coat. Technol. 187, 284–292 (2004)
J. Arana et al., TiO2 activation by using activated carbon as a support: surface characterization and decantability study. Appl. Catal. B-Environ. 44, 161–172 (2003)
B. Tryba, T. Tsumura, M. Janus, A.W. Morawski, M. Inagaki, Carbon-coated anatase: adsorption and decomposition of phenol in water. Appl. Catal. B-Environ. 50, 177–183 (2004)
K.F. Zhou, Y.H. Zhu, X.L. Yang, X. Jiang, C.Z. Li, Preparation of graphene–TiO2 composites with enhanced photocatalytic activity. New J. Chem. 35, 353–359 (2011)
K. Woan, G. Pyrgiotakis, W. Sigmund, Photocatalytic carbon canotube–TiO2 composites. Adv. Mater. 21, 2233–2239 (2009)
B. Gao, G.Z. Chen, G.L. Puma, Carbon nanotubes–titanium dioxide (CNTs–TiO2) nanocomposites prepared by conventional and novel surfactant wrapping sol–gel methods exhibiting enhanced photocatalytic activity. Appl. Catal. B-Environ. 89, 503–509 (2009)
A. Leaustic, F. Babonneau, J. Livage, Structural investigation of the hydrolysis-condensation process of titanium alkoxides Ti(OR)4 (OR = OPri, OEt) modified by acetylacetone. 1. Study of the alkoxide modification. Chem. Mat. 1, 240–247 (1989)
A. Leaustic, F. Babonneau, J. Livage, Structural investigation of the hydrolysis-condensation process of titanium alkoxides Ti(OR)4 (OR = OPri, OEt) modified by acetylacetone. 2. From the modified precursor to the colloids. Chem. Mat. 1, 248–252 (1989)
Y.S. Ho, G. McKay, Sorption of dye from aqueous solution by peat. Chem. Eng. J. 70, 115–124 (1998)
N. Satoh, T. Nakashima, K. Kamikura, K. Yamamoto, Quantum size effect in TiO2 nanoparticles prepared by finely controlled metal assembly on dendrimer templates. Nat. Nanotech. 3, 106–111 (2008)
A. Kataee, G.A. Mansoori, Nanostructured Titanium Dioxide Materials: Properties, Preparation And Applications (World Scientific Publishing Co Pte Ltd., Singapore, 2012)
S. Sajjad, Synthesis, characterization and applications of nanomaterials in the field of photocatalysis. Doctoral Thesis/Dissertation, 2011
Acknowledgments
This work was supported by the Capacity Building Program of Shanghai Local Universities (No. 12160503600), the First-Class Discipline Construction Fund of Shanghai Municipal Education Commission (No. J201212), Nature Science Foundation of China (U1332107) and Key Discipline Construction Fund of Composite Materials of Shanghai Institute of Technology (No. 10210Q140001).
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, X., Zhu, L., Pan, F. et al. Effects of complexing ligand in sol–gel process on the photocatalytic activity of TiO2–C hybrid aerogels. Appl. Phys. A 119, 1459–1467 (2015). https://doi.org/10.1007/s00339-015-9120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9120-5