Abstract
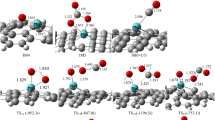
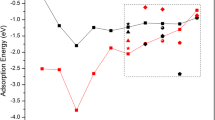
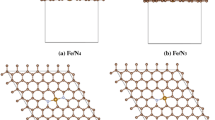
The geometric stability, electronic structure and catalytic activity of the Mo-embedded graphene (Mo/SV-graphene) are investigated by using the first-principle calculations. Compared with a Mo atom on pristine graphene, the Mo dopant in defective graphene exhibits more positively charged, which helps to weaken the CO adsorption and facilitates the O2 adsorption. Besides, the two mechanisms (Langmuir–Hinshelwood, LH and Eley–Rideal, ER) for the sequential CO oxidation reactions are investigated comparison. Among the reaction processes, the coadsorption of O2 and CO exists at the Mo/SV-graphene surface, the first step (CO + O2 → OOCO) with energy barrier is 0.60 eV and then form a CO2 molecule through the reaction (OOCO → CO2 + Oads) without any energy barrier, and thus the formation of OOCO complex is viewed as rate-controlling step. In the ER reaction, although the CO molecule reacts with the preadsorbed O2 by the low-energy barrier (CO + O2 → CO3, 0.13 eV), the formation of CO3 is more stable than the generating CO2 and Oads (0.84 eV). Hence, the LH mechanism as the starting step is energetically more favorable. The results provide the valuable guidance to fabricate graphene-based catalysis and validate the reactivity of atomic-scale catalyst.







Similar content being viewed by others
References
A.A. Herzing, C.J. Kiely, A.F. Carley, P. Landon, G.J. Hutchings, Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 321, 1331 (2008)
A. Hornés, A. Hungría, P. Bera, A.L. Cámara, M. Fernández-García, A. Martínez-Arias, L. Barrio, M. Estrella, G. Zhou, J. Fonseca, Inverse CeO2/CuO catalyst as an alternative to classical direct configurations for preferential oxidation of CO in hydrogen-rich stream. J. Am. Chem. Soc. 132, 34 (2009)
S.N. Rashkeev, A.R. Lupini, S.H. Overbury, S.J. Pennycook, S.T. Pantelides, Role of the nanoscale in catalytic CO oxidation by supported Au and Pt nanostructures. Phys. Rev. B 76, 035438 (2007)
A. Eichler, CO oxidation on transition metal surfaces: reaction rates from first principles. Surf. Sci. 498, 314 (2002)
N. Lopez, J.K. Nørskov, Catalytic CO oxidation by a gold nanoparticle: a density functional study. J. Am. Chem. Soc. 124, 11262 (2002)
W. Wallace, R. Whetten, Coadsorption of CO and O2 on selected gold clusters: evidence for efficient room-temperature CO2 generation. J. Am. Chem. Soc. 124, 7499 (2002)
U. Heiz, A. Sanchez, S. Abbet, W.D. Schneider, Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: each atom counts. J. Am. Chem. Soc. 121, 3214 (1999)
K. Bleakley, P. Hu, A Density Functional Theory Study of the Interaction between CO and O on a Pt Surface: CO/Pt (111), O/Pt (111), and CO/O/Pt (111). J. Am. Chem. Soc. 121, 7644 (1999)
S. Li, Z. Lu, Z. Yang, X. Chu, The sensing mechanism of Pt-doped SnO2 surface toward CO: a first-principle study. Sens. Actuator B Chem. 202, 83 (2014)
X.Q. Gong, Z.P. Liu, R. Raval, P. Hu, A systematic study of CO oxidation on metals and metal oxides: density functional theory calculations. J. Am. Chem. Soc. 126, 8 (2004)
A. Zhang, J. Zhu, W. Duan, Oxidation of carbon monoxide on Rh (111): a density functional theory study. J. Chem. Phys. 124, 234703 (2006)
R. Kou, Y. Shao, D. Mei, Z. Nie, D. Wang, C. Wang, V.V. Viswanathan, S. Park, I.A. Aksay, Y. Lin, Stabilization of electrocatalytic metal nanoparticles at metal–metal oxide–graphene triple junction points. J. Am. Chem. Soc. 133, 2541 (2011)
E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura, I. Honma, Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nanoletters 9, 2255 (2009)
K. Novoselov, A. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva, A. Firsov, Electric field effect in atomically thin carbon films. Science 306, 666 (2004)
K. Novoselov, A. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos, A. Firsov, Two-dimensional gas of massless Dirac fermions in graphene. Nature 438, 197 (2005)
C. Lee, X. Wei, J.W. Kysar, J. Hone, Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321, 385 (2008)
J. Zhang, S. Tang, L. Liao, W. Yu, J. Li, F. Seland, G.M. Haarberg, Improved catalytic activity of mixed platinum catalysts supported on various carbon nanomaterials. J. Power Sources 267, 706 (2014)
E. Yoo, T. Okada, T. Akita, M. Kohyama, I. Honma, J. Nakamura, Sub-nano-Pt cluster supported on graphene nanosheets for CO tolerant catalysts in polymer electrolyte fuel cells. J. Power Sources 196, 110 (2011)
G. Kim, S.H. Jhi, Carbon monoxide-tolerant platinum nanoparticle catalysts on defect-engineered graphene. ACS Nano 5, 805 (2011)
Y. Lu, M. Zhou, C. Zhang, Y. Feng, Metal-embedded graphene: a possible catalyst with high activity. J. Phys. Chem. C 113, 20156 (2009)
E.H. Song, Z. Wen, Q. Jiang, CO catalytic oxidation on copper-embedded graphene. J. Phys. Chem. C 115, 3678 (2011)
Y.N. Tang, Z.X. Yang, X.Q. Dai, A theoretical simulation on the catalytic oxidation of CO on Pt/graphene. Phys. Chem. Chem. Phys. 14, 16566 (2012)
T.T. Jia, C.H. Lu, Y.F. Zhang, W.K. Chen, A comparative study of CO catalytic oxidation on Pd-anchored graphene oxide and Pd-embedded vacancy graphene. J. Nanopart. Res. 16, 1 (2014)
A. Krasheninnikov, P. Lehtinen, A. Foster, P. Pyykkö, R. Nieminen, Embedding transition-metal atoms in graphene: structure, bonding, and magnetism. Phys. Rev. Lett. 102, 126807 (2009)
Q. Tang, Z. Zhou, Z. Chen, Graphene-related nanomaterials: tuning properties by functionalization. Nanoscale 5, 4541 (2013)
Y. Li, Z. Zhou, G. Yu, W. Chen, Z. Chen, CO catalytic oxidation on iron-embedded graphene: computational quest for low-cost nanocatalysts. J. Phys. Chem. C 114, 6250 (2010)
F. Li, J. Zhao, Z. Chen, Fe-anchored graphene oxide: a low-cost and easily accessible catalyst for low-temperature CO oxidation. J. Phys. Chem. C 116, 2507 (2012)
A. Ambrosi, S.Y. Chee, B. Khezri, R.D. Webster, Z. Sofer, M. Pumera, Metallic impurities in graphenes prepared from graphite can dramatically influence their properties. Angew. Chem. Int. Ed. 51, 500 (2012)
G. Kresse, J. Furthmüller, Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15 (1996)
G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996)
G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999)
J. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996)
J. Carlsson, M. Scheffler, Structural, electronic, and chemical properties of nanoporous carbon. Phys. Rev. Lett. 96, 46806 (2006)
G. Henkelman, A. Arnaldsson, H. Jónsson, A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354 (2006)
G. Henkelman, B. Uberuaga, H. Jónsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901 (2000)
G. Henkelman, H. Jónsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978 (2000)
T. Zhu, J. Li, S. Yip, Atomistic study of dislocation loop emission from a crack tip. Phys. Rev. Lett. 93, 25503 (2004)
Y.N. Tang, Z.X. Yang, X.Q. Dai, Noble metals induced magnetic properties of graphene. J. Magn. Magn. Mater. 323, 2441 (2011)
Y.N. Tang, Z.X. Yang, X.Q. Dai, D.W. Ma, Z.M. Fu, Formation, stabilities, and electronic and catalytic performance of platinum catalyst supported on non-metal-doped graphene. J. Phys. Chem. C 117, 5258 (2013)
L.M. Molina, B. Hammer, The activity of the tetrahedral Au20 cluster: charging and impurity effects. J. Catal. 233, 399 (2005)
W. An, Y. Pei, X.C. Zeng, CO oxidation catalyzed by single-walled helical gold nanotube. Nanoletters 8, 195 (2008)
Y.N. Tang, X.Q. Dai, Z.X. Yang, Z.Y. Liu, L.J. Pan, D.W. Ma, Z.S. Lu, Tuning the catalytic property of non-noble metallic impurities in graphene. Carbon 71, 139 (2014)
Q.G. Jiang, Z.M. Ao, S. Li, Z. Wen, Density functional theory calculations on the CO catalytic oxidation on Al-embedded graphene. RSC Adv. 4, 20290 (2014)
A. Alavi, P. Hu, T. Deutsch, P.L. Silvestrelli, J. Hutter, CO oxidation on Pt (111): an ab initio density functional theory study. Phys. Rev. Lett. 80, 3650 (1998)
M. Ackermann, T. Pedersen, B. Hendriksen, O. Robach, S. Bobaru, I. Popa, C. Quiros, H. Kim, B. Hammer, S. Ferrer, J.W.M. Frenken, Structure and reactivity of surface oxides on Pt (110) during catalytic CO oxidation. Phys. Rev. Lett. 95, 255505 (2005)
Y.N. Tang, Z.X. Yang, X.Q. Dai, Z.S. Lu, Y.X. Zhang, Z.M. Fu, Theoretical study of the catalytic CO oxidation by Pt catalyst supported on Ge-doped graphene. J. Nanosci. Nanotechnol. 14, 7117 (2014)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. U1404109 51401078 and 11247012), and the Science Fund of Educational Department of Henan Province (Grant Nos. 14B140019, 14A140015 and 14A140010).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, Y., Pan, L., Chen, W. et al. Reaction mechanisms for CO catalytic oxidation on monodisperse Mo atom-embedded graphene. Appl. Phys. A 119, 475–485 (2015). https://doi.org/10.1007/s00339-015-9093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9093-4