Abstract
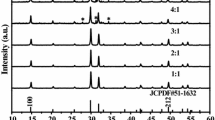
A series of double molybdates phosphors AEu(MoO4)2 (A = Li, Na, K and Ag) have been prepared by sol-gel method. Their crystal structure and luminescent properties have also been investigated in a comparable way. The crystallization processes of the phosphor precursors were characterized by X-ray diffraction (XRD) and thermogravimetry-differential thermal analysis (TG-DTA). Field emission scanning electron microscopy (FE-SEM) was also used to characterize the shape and size distribution of the phosphors. Samples except KEu(MoO4)2 showed tetragonal scheelite structure in the range of our experiments, and no phase transition appeared. Phosphor KEu(MoO4)2 possessed two structures, and the phase transition took place at about 800°C. All samples with high purity could be obtained at about 500°C for 5 hours, and they all showed intense red light peaked at 616 nm originated from 5D0→7F2 emission of Eu3+ under the excitation of 465 nm or 394 nm light. The excitation spectra of phosphors AEu(MoO4)2 (A = Li, Na, and K) are composed of a strong broad charge transfer (CT) band and some sharp lines, and the relative intensity of CT band, the two strongest absorption lines at 395 nm and 465 nm are comparative, so these three phosphors are good red phosphor candidates for violet or blue LEDs. For the excitation spectrum of phosphor AgEu(MoO4)2, intensities of CT band and the absorption line at 395 nm are much weaker than that of line at 465 nm, thus phosphor AgEu(MoO4)2 is only suit for GaN-based blue LED.
Similar content being viewed by others
References
Z. Wang, H. Liang, J. Wang, M. Gong, Q. Su, Appl. Phys. Lett. 89, 071921 (2006)
C.-H. Chiu, C.-H. Liu, S.-B. Huang, T.-M. Chen, J. Electrochem. Soc. 154(7), J181–J184 (2007)
M. Zachau, D. Berben, T. Fiedler, F. Jermann, F. Zwaschka, Proc. SPIE 6910, 691010-1 (2008)
J. Edmond, A. Abare, M. Bergman, J. Bharathan, K.L. Bunker, D. Emerson, K. Haberern, J. Ibbetson, M. Leung, P. Russel, D. Slater, J. Cryst. Growth 272, 242–250 (2004)
H.S. Jang, W.B. Im, D.C. Lee, D.Y. Jeon, S.S. Kim, J. Lumin. 126, 371–377 (2007)
R. Mueller-Mach, G. Mueller, M.R. Krames, H.A. Hoppe, F. Stadler, W. Schnick, T. Juestel, P. Schmidt, Phys. State Solid (a) 202(9), 1727–1732 (2005)
C. Guo, D. Huang, Q. Su, Mater. Sci. Eng. B 130, 189–193 (2006)
C. Guo, W. Zhang, L. Luan, C. Tao, H. Cheng, D. Huang, Sens. Actuators B: Chem. (2008). doi:10.1016/j.snb.2008.01.065
Z. Wang, H. Liang, M. Gong, Q. Su, Opt. Mater. 29(7), 896–900 (2007)
F. Shi, J. Meng, Y. Ren, J. Solid State Chem. 121, 236–239 (1996)
J.P.M. Van Vliet, G. Blasse, L.H. Brixner, J. Solid State Chem. 76, 160–166 (1988)
H. Winkler, H. Enderle, C. Kuehn, R. Petry, T. Vosgroene, Proc. SPIE 6797, 67970A (2007)
H. Zhang, M. Lv, Z. Xiu, S. Wang, G. Zhou, Y. Zhou, S. Wang, Z. Qiu, A. Zhang, Mater. Res. Bull. 42, 1145–1152 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, C., Wang, S., Chen, T. et al. Preparation of phosphors AEu(MoO4)2 (A = Li, Na, K and Ag) by sol-gel method. Appl. Phys. A 94, 365–371 (2009). https://doi.org/10.1007/s00339-008-4811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-008-4811-9