Abstract
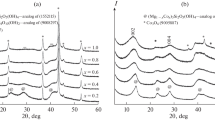
Hydrofullerides with hydrogen content up to 5 wt. % were obtained by direct and catalytic reactions with H2 gas. Hydrogen content was monitored ‘in situ’ using a gravimetric system, and verified by chemical analysis ‘ex situ’. It was found that pure C60 reacts rapidly when exposed to H2 gas at 673 K and 50–100 bar. Gravimetric study of this reaction showed that hydrogenation is saturated at about 5 wt. % of hydrogen. The mass of the sample goes through a maximum and with a longer reaction time its weight starts to decrease. This proves that hydrofullerides with high hydrogen content are not stable and strong hydrogenation results in the collapse of C60 molecules. XRD studies showed that samples prepared by direct hydrogenation without a catalyst retain an original fcc structure with an increase of the cell parameter a up to 15.1 Å. Catalytic hydrogenation of C60 with H2 gas results in a decrease of the reaction temperature and formation of hydrofullerides with different types of crystal structures.
Similar content being viewed by others
References
R.O. Loufty, E.M. Wexler: Procs. of 2001 DOE Hydrogen Prog. Rev. NREL/CP-570-30535
B.P. Tarasov, Y.M. Shul’ga, V.N. Fokina, V.N. Vasilets, N.Y. Shul’ga, D.V. Schur, V.A. Yartys: J. Alloys and Compounds 314, 296 (2001)
Y.M. Shul’ga, B.P. Tarasov, V.N. Fokin, V.M. Martynenko, D.V. Schur, G.A. Volkov, V.I. Rubtsov, G.A. Krasochka, N.V. Chapusheva, W. Shevchenko: Carbon 41, 1365 (2003)
A.D. Darwish, A.K. Abdul-Sada, G.J. Langley, H.W. Kroto, R. Taylor, D.R.M. Walton: Synth.Metals 77, 303 (1996)
C. Ruchardt, M. Gerst, J. Ebenhoch, H.D. Beckhaus, E.E.B. Campbell, R. Tellgmann, H. Schwarz, T. Weiske, S. Pitter: Angew. Chem. 105, 609 (1993)
A.I. Kolesnikov, V.E. Antonov, I.O. Bashkin, G. Grosse, A.P. Moravsky, A.Y. Muzychka, E.G. Ponyatovsky, F.E. Wagner: J. Phys. Condens. Matter 9, 2831 (1997)
Y.M. Shul’ga, B.P. Tarasov, V.N. Fokin, N.Y. Shul’ga, V.N. Vasilets: Fiz. Tv. Tela 41, 1520 (1999)
A.V. Okotrub, L.G. Bulusheva, I.P. Acing, A.S. Lobach, Y.M. Shulga: J. Phys. Chem. 103, 716 (1999)
A.D. Darwish, A.K. Abdul-Sada, G.J. Langley, H.W. Kroto, R. Taylor, D.R.M. Walton: Chem. Soc., Perkin Trans. 2, 2359 (1995)
K. Balasubramanian: Am. Chem. Soc. 114, 7300 (1992)
V.N. Bezmelnitsyn, V.P. Glazkov, V.P. Zhukov, V.A. Somenkov, S.S. Shilstein: In: The 4th Biennial International Workshop of Russia Clusters (Abstracts of Reports). St. Petersburg, 1999, p. 71
W. Kockelmann: ISIS exper. rep., Rutherford Appleton Lab., (2000)
L.E. Halle, D.R. McKenzie, M.I. Attalla, A.M. Vassallo, R.L. Davis, J.B. Dunlop, D.J.H. Cockayne: J. Phys. Chem. 97, 5741 (1993)
V.E. Antonov, I.O. Bashkin, S.S. Khasanov, A.P. Moravsky, Y.G. Morozov, Y.M. Shulga, Y.A. Ossipyan, E.G. Ponyatovsky: J. Alloys Compd. 330–332, 365 (2002)
A. Rathna, J. Chandrasekhar: Chem. Phys. Lett. 206, 217 (1993)
M.I. Attala, A.M. Vassalo, B.H. Tattam, J.V. Hanna: J. Phys. Chem. 97, 6329 (1993)
R. Bini, J. Ebenhoch, M. Fanti, P.W. Fowler, S. Leach, G. Orlandi, C. Ruchardt, J.P.B. Sandall, F. Zerbetto: Chem. Phys. 232, 75 (1998)
L.G. Bulisheva, A.V. Okotrub, A.V. Antich, A.S. Lobach: J. Mol. Structure 562, 119 (2001)
B.P. Tarasov, V.N. Fokin, E.E. Fokina, Z.A. Rumynskaya, L.S. Volkova, A.P. Moravskii, Y.M. Shul’ga: Russ. J. Gen. Chem. 68, 1515 (1998)
R. Tycko, R.C. Haddon, G. Dabbagh, S.H. Glarum, D.C. Douglass, A.M. Mujse: J. Phys. Chem. 95, 518 (1991)
P.A. Dorozko, A.S. Lobach, A.A. Popov, V.M. Senyavin, M.V. Korobov: Chem. Phys. Lett. 336, 39 (2001)
J.E. Fischer, P.A. Heiney, A.B. Smith: Acc. Chem. Res. 25, 112 (1992)
W.I.F. David, R.M. Ibberson, J.C. Matthewman, K. Prassides, T.J.S. Dennis, J.P. Hare, H.W. Kroto, R. Taylor, D.R.M. Walton: Nature 353, 147 (1991)
P.A. Heiney, G.B.M. Vaughan, J.E. Fischer, N. Coustel, D.E. Cox, J.R.D. Copley, D.A. Neumann, W.A. Kamitakahara, K.M. Creegan, D.M. Cox, J.P. McCauley, Jr., A.B. Smith III: Phys. Rev. B 45, 4544 (1992)
Author information
Authors and Affiliations
Corresponding author
Additional information
PACS
61.48.+c; 61.10.Nz; 81.05.Tp
Rights and permissions
About this article
Cite this article
Talyzin, A., Shulga, Y. & Jacob, A. Comparative study of hydrofullerides C 60 H x synthesized by direct and catalytic hydrogenation. Appl. Phys. A 78, 1005–1010 (2004). https://doi.org/10.1007/s00339-003-2422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-003-2422-z