Abstract
Sexual propagation of corals is a promising strategy for coral restoration, but one of the main challenges is the high mortality of coral spat due to competitive interactions with macroalgae during the early life history stages. Optimising the properties of settlement substrates such as material types and surface roughness has the potential to improve the survival of spat by limiting the recruitment and growth of macroalgae. In this study, we assessed the effects of modifying surface roughness across three different tile materials (alumina-based ceramic, calcium carbonate (CaCO3), and concrete) on the settlement success and post-settlement survivorship of Acropora kenti coral larvae in six mesocosm tanks, each with different established macroalgal communities. The macroalgal community compositions on the tiles were significantly different among material types, but not surface roughness, although the type and abundance of macroalgal species were heavily influenced by the established tank communities. Increasing surface roughness did not affect larval settlement success or spat survivorship. Substantially higher larval settlement density was found on concrete tiles (1.92 ± 0.10 larvae cm−2), but spat survival was the highest on CaCO3 tiles (73.4 ± 4.2% survived). Very strong competitive interactions were observed between spat and macroalgae, with overgrowth by the crustose coralline alga Crustaphytum sp. and the brown alga Lobophora sp. being the primary cause of spat mortality. Overall, when taking into account both settlement and survival rates, concrete was the best performing among the tile types tested here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global decline in coral populations, as a result of ocean warming and local anthropogenic stressors, have prompted active restoration efforts to accelerate the recovery of coral reefs and enhance their resilience (Boström-Einarsson et al. 2020; Banaszak et al. 2023). One key intervention is sexual propagation, which harnesses the ability of relatively few corals to produce millions of offspring annually (Boström-Einarsson et al. 2020; Randall et al. 2020). Recent studies have also applied novel methods to ‘seed’ sexually propagated corals on coral reefs by settling coral larvae on artificial substrates ex situ and attaching them to devices to be deployed onto reefs (Chamberland et al. 2017; Randall et al. 2023). Compared to the traditional asexual propagation methods, coral seeding is more ecologically sustainable as it does not require the sacrifice of the parental colonies (Randall et al. 2020). Coral seeding also maintains the genetic diversity in the outplanted corals, with the potential to survive and adapt to changing environmental conditions (van Oppen et al. 2017; Baums et al. 2019).
One of the main bottlenecks in coral seeding is the high mortality of corals during their early life-history stages (i.e., type III survival; Vermeij and Sandin 2008), particularly in newly settled coral larvae—termed as ‘spat’ hereafter. While high larval settlement can be achieved in coral seeding, majority of spat tend not to survive beyond six months (Edwards et al. 2015; Randall et al. 2021). The main causes of mortality in spat include predation, sediment smothering, and competition with other benthic organisms, predominantly macroalgae (Babcock and Smith 2002; Penin et al. 2011; Doropoulos et al. 2016). While some macroalgae, in particular crustose coralline algae (CCA), may be desirable during the settlement process as they can provide chemical cues to induce coral larval settlement (Heyward and Negri 1999; Tebben et al. 2015; Abdul Wahab et al. 2023) and limit establishment of other macroalgae (Vermeij et al. 2011; Gomez-Lemos and Diaz-Pulido 2017), fleshy macroalgae generally have detrimental impacts on corals during the post-settlement period (reviewed by Birrell et al. 2008a). Macroalgae can reduce spat growth and survival by means of physical mechanisms such as space pre-emption, overgrowth, and abrasion (River and Edmunds 2001; Vermeij 2006); allelopathy (Kuffner et al. 2006; Paul et al. 2011); and alteration of the micro-environment including light and oxygen availability, water flow, sediment and nutrient regimes, as well as microbial communities (Box and Mumby 2007; Hauri et al. 2010; Wangpraseurt et al. 2012; Bulleri et al. 2018).
To improve the effectiveness and scale of seeding sexually propagated corals in restoration efforts, it is critical to select settlement substrates that optimise the settlement success of coral larvae and their early post-settlement survival (Banaszak et al. 2023). Previous studies have used a range of materials as settlement substrates for coral larvae, including concrete, ceramic, terracotta, coral skeleton, and plastics such as PVC (Spieler et al. 2001; Burt et al. 2009; Antink et al. 2018; Leonard et al. 2022). Physical and chemical characteristics of the substrates such as surface topography, porosity, and chemical composition can influence the settlement preference of macroalgae and recruitment success of coral larvae (Fletcher and Callow 1992; Diaz-Pulido and McCook 2004; Petersen et al. 2005a; Whalan et al. 2015; Levenstein et al. 2022). In particular, macroalgal spores may have higher settlement rates on substrates with surface microtopography that matches their cell sizes to achieve maximum contact points (Scardino et al. 2008). However, most studies to date have tested the effectiveness of surface roughness in inhibiting the settlement of macroalgal propagules in monospecific experiment assays and did not examine its potential impacts on early coral recruitment success (Callow et al. 2002; Schumacher et al. 2007; Xiao et al. 2018). It remains unclear whether modifying surface roughness will be useful in controlling the growth of multispecies macroalgal communities to improve the survival of coral spat in aquaculture for restoration purposes.
In this study, the roles of material type and surface roughness of settlement tiles on the early recruitment success of Acropora kenti coral larvae were evaluated in a coral aquaculture facility. Specifically, we assessed how the establishment, abundance and composition of macroalgal communities varied with increasing surface roughness in alumina-based ceramic, calcium carbonate (CaCO3), and concrete tiles (prior to and post coral settlement). We compared the settlement success of coral larvae among the tile treatments and subsequently assessed the post-settlement survivorship and growth of spat over 10 weeks. We also quantified the frequency of competitive interactions between spat and macroalgal taxa to better understand the role of coral–macroalgal interactions in mediating spat survivorship.
Materials and methods
To examine the effects of material types and surface roughness of settlement tiles on macroalgal community composition and early coral recruitment success, we conducted a mesocosm experiment in indoor aquaria at the National Sea Simulator (SeaSim) of the Australian Institute of Marine Science (AIMS) in Townsville, Australia. We tested three material types (alumina, CaCO3, and concrete) and five surface roughness levels of settlement tiles (82 mm × 90 mm). In total, there were 15 types of tiles. Tiles were first conditioned for six weeks to allow mixed communities of biofilm and macroalgae to develop, followed by a coral larval settlement choice assay and 10 weeks monitoring of post-settlement survival of coral spat (Fig. 1).
Schematic diagram of the experimental design illustrating: (1) the 6-week tile conditioning in the 280-L mesocosm tanks, followed by (2) larval settlement substrate choice assay and subsequent symbiont inoculation in the 50-L settlement tanks, and (3) the post-settlement monitoring of spat survival in the 280-L mesocosm tanks for 10 weeks
Material types and surface roughness of settlement tiles
Settlement tiles were comprised of three different material types, namely alumina, CaCO3, and concrete. Alumina (Al2O3) is an inert ceramic with high hardness that is very resistant to erosion and corrosion. CaCO3 tiles were included as coral reef frameworks are comprised of CaCO3 deposited mainly by corals and crustose coralline algae. Finally, concrete was included as it is one of the most widely used materials in coral recruitment studies and reef restoration projects due to its low cost, ready availability, and common use in marine construction (Spieler et al. 2001; Burt et al. 2009; Chamberland et al. 2017).
Fully sintered 95% pure alumina tiles were sourced from Gongtao Ceramics (Shanghai, People’s Republic of China) and were used with no further modification as Roughness 1, while partially sintered alumina tiles sourced from Alpha Ceramics GmbH (Aachen, Germany) were sand blasted to modify their surface roughness (Table 1). Fully sintered alumina was obtained by sintering the tiles at 1600 °C while partially sintered alumina tiles were sintered at 1200 °C, achieving a density of 2.42 g cm−3 (63% densification). CaCO3 tiles were fabricated from casting CaCO3 powder (Oplusi, USA), which was initially dispersed in water using polyvinyl alcohol (PVA) binder. Fabricated CaCO3 tiles were chosen over coral rubble slabs to improve sustainability and allow better manipulation of the surface roughness of the tiles. Concrete tiles were prepared following the standard AS 2350.12–2006 Sect. 6 and 7 (Standards Australia 2016) and the surface roughness of the tiles was modified by sandblasting (Table 1).
The surface roughness of the tiles was characterized using laser scanning microscopy (Olympus LEXT OLS4100) by randomly scanning five areas (2.583 × 2.574 cm) to obtain the 3D surface profile images. Three metrics of areal surface roughness were calculated following the ISO 25178 “Geometrical product specification (GPS)—Surface texture: Areal”: (1) Sa—the arithmetical mean height of the sample profile, (2) Sp—the maximum peak height within the sampling area, and (3) Sv—the maximum valley depth within the sampling area. All three metrics had increasing values with increasing roughness levels, except for CaCO3 tiles which had lower Sa values at roughness 4 and 5 compared to roughness 3 (Fig. 2). Tiles with higher surface roughness also generally had a higher degree of irregularity and waviness in both the x and y planes (ESM Fig. S1).
The chemical composition of the tiles was confirmed using scanning electron microscope (Jeol JSM5410LV) coupled with energy-dispersive X-ray spectroscopy (ESM Fig. S2). As expected, alumina tiles were largely comprised of aluminium (53 wt%) and oxygen (47 wt%), while the concrete tiles were composed mainly of calcium (39 wt%) and oxygen (37 wt%). The main constituents in the CaCO3 tiles were calcium (47 wt%) and oxygen (34 wt%).
Experimental designs
Thirty tiles of each of the 15 tile types were distributed across six 280-L fiberglass mesocosm tanks (L 1400 mm × W 755 mm × H 260 mm). In total, 450 tiles were tested (3 material × 5 roughness × 5 replicate × 6 tanks). As each mesocosm tank had established fouling communities with different macroalgal community compositions, we replicated the experiment in six tanks. Established mesocosm tanks are preferred over bare tanks for raising coral spat as tiles maintained in bare tanks often become rapidly dominated by different filamentous and turf algal communities, which require more husbandry (Petersen et al. 2005b; Neil et al. 2021). However, the macroalgal communities are always (at least subtly) different between established mesocosm tanks; thus, to represent a variety of possible macroalgal communities, we replicated the experiment across six independent tanks. While this approach introduces variability in macroalgal colonisation on settlement tiles, it is largely unavoidable and has the advantage that any significant effects of substrate treatments (i.e., material and roughness) on spat settlement and survival are robust across macroalgal communities. This also likely broadens the application of results in future coral aquaculture programs that utilise already established mesocosms. Tiles were placed on angled PVC racks that kept the tiles at an angle of 38° relative to the tank floor to reduce the potential effects of sediment deposition on the macroalgal communities and newly settled spat. Positions of the tiles in each tank were randomized every two weeks to minimize any positional effects.
All mesocosm tanks had a semi-recirculation system with independent sumps and an average of 300% turnover per day. Each tank was fitted with two LED panel lights that followed a sinusoidal profile of ramping from darkness at 05:30 to a maximum of ~ 50 μmol quanta m−2 s−1 (PAR) at 12:00 and then a ramp-down to darkness at 18:30, achieving daily light integral of 1.4 mol m−2 d−1. This light level was selected because it was the optimal intensity for CCA conditioning in the SeaSim (B. Ramsby, unpublished data). Water temperature followed the historical profile obtained from the daily mean temperature at 4 m depth at Davies Reef (18°49′53″S 147°38′8″E) between 1991 and 2012 (ESM Fig. S3). Both temperature and light were controlled by a Supervisory Control and Data Acquisition (SCADA) system (Siemens PCS7). Two gyre pumps (Maxspect 350 series) were used to maintain the water circulation in each tank. At the start of the experiment, 10 Turbo sp. (5–12 mm shell) and 8 Calthalotia sp. (1–4 mm shell) snails were added to each tank. These small grazing herbivores have been shown to help control macroalgal growth and improve coral spat survivorship in the aquarium facilities (Neil et al. 2021). Water quality parameters were measured every two weeks and no anomalies were recorded (NO2−: 0.079 ± 0.002 µmol L−1, mean ± SE; NO3−: 2.119 ± 0.158 µmol L−1; NH4+: 0.169 ± 0.005 µmol L−1; PO4−: 0.174 ± 0.005 µmol L−1; SiO2: 6.661 ± 0.436 µmol L−1).
To account for potential factors driving the tank differences, we characterized the established macroalgal communities in each mesocosm tank by placing six permanent 10 cm × 10 cm quadrats along the inner walls of each tank. Quadrats were photographed (Canon PowerShot S120) at the start of the experiment, at the end of six weeks of tile conditioning, and at the end of 10 weeks of post-settlement monitoring. Macroalgal community composition was assessed from the photographs using the Coral Point Count with Excel extension (CPCe; Kohler and Gill 2006), whereby 25 stratified random points were overlaid on each quadrat and the macroalgal taxa (genus-level) directly under the points were identified. Identification of macroalgal species was based on their morphological and anatomical features examined under stereo- and light microscopes and using taxonomic literature (e.g., Price and Scott 1992; Kraft 2007; Huisman 2015).
Coral collection, spawning, and larval culture
Gravid colonies of Acropora kenti were collected from reefs around the Palm Island Group, Australia (18°45′56.4″S 146°32′2.58″E) in October 2022, one week prior to the full moon (GBRMPA Permit G21/45348.1). Recent molecular studies by Bridge et al. (2023) revealed the cryptic diversity within the ‘Acropora tenuis’ species complex and discovered that species that was previously referred to as A. tenuis on the GBR and elsewhere in eastern Australia was actually A. kenti. However, the species used in the current study, common to the Palm Island group, has some morphological deviations from the A. kenti voucher specimen and is therefore referred to as Acropora sp. nov. aff. kenti but abbreviated to A. kenti.
Colonies were transported to the SeaSim and kept in outdoor holding tanks (1200 L; L 2800 mm × W 1000 mm × H 440 mm) until spawning. Six days following the full moon, 16 colonies spawned, and their gametes were collected and pooled for cross fertilisation. Larvae were cultured in 500-L flow-through tanks at densities of ~0.6 larva mL−1 until they achieved settlement competency. Daily competency assays (Heyward and Negri 1999) showed that 5-day-old A. kenti larvae were competent, attaining >90% settlement rate in the presence of the CCA Porolithon cf. onkodes fragments.
Coral larval settlement substrate choice
To examine whether coral larval settlement success varied among material types and surface roughness of the settlement tiles, we conducted settlement choice assays in eighteen 50-L flow-through acrylic tanks (L 600 mm × W 300 mm × H 330 mm). Each tank contained one of each 15 tile types from the same mesocosm tank; three replicates per tile type from each mesocosm tank were assayed. Approximately 2,700 A. kenti larvae (5-day-old) were added to each settlement tank, where they had equal opportunity to settle on each tile type. To prevent larvae from settling on the side and underside of the tiles, each tile was surrounded by sterile glass beads (ϕ 2–3 mm) such that only the top surface of the tile was presented to the larvae. The sides of each tile were also sanded to remove any macroalgae prior to the settlement choice assays. The assays were performed under 35 PAR (12 h light: 12 h dark cycle) at 27.5 °C.
After 24 h, the tiles were photographed using a Nikon D810 camera (Nikon AF-S 60 mm f/2.8G ED macro lens with two Ikelite DS161 strobes) to determine (1) the number of coral larvae that successfully settled and metamorphosed, and (2) the macroalgal community composition. Coral larvae were scored as settled if they were firmly attached to the substrate with pronounced flattening of the oral-aboral axis and septal mesenteries radiating from the central mouth (Heyward and Negri 1999). The macroalgal community composition on each tile was estimated using the CPCe software in the same manner as the tank community assessments.
Coral spat survivorship
Due to a low number of spat (<20) on some of the tiles, another 2,700 A. kenti larvae (6-day-old) were added to each of the settlement tanks to even out the number of spat across the treatments. Tiles were photographed after 24 h to determine the number of spat. Spat were kept in the settlement tank assays for another 65 h for inoculation with cultured Symbiodiniaceae that were originally isolated from A. kenti from Magnetic Island, Australia (1.54 × 108 cells of Cladocopium proliferum added to each settlement tank, ID: 055–01.10; Butler et al. 2023). All tiles were then returned to their respective mesocosm tanks and fed once daily (1600 h) with 2,000 cells mL−1 microalgae (Tisochrysis lutea, Nannochloropsis oceanica, Pavlova lutheri, Dunaliella sp.) and 0.5 nauplii mL−1 rotifers.
Tiles were photographed regularly (week 1, 4, 7, and 10 post-settlement) to monitor the macroalgal communities, spat survivorship, spat growth, and the interactions between spat and macroalgae. As little differences in the macroalgal communities and coral settlement rates were observed across the five surface roughness categories during the settlement choice assays (Figs. 3a and 5a), we only focused on roughness 1, 3, and 5 to represent the increasing roughness levels for the post-settlement monitoring. Preliminary analyses also revealed no effect of surface roughness on spat survivorship, so no further data collection was performed for roughness 2 and 4. Spat that formed aggregates during settlement or fused with neighbouring spat during the grow-out period were excluded from the dataset because aggregates would have confounded spat survival and growth rates (Ligson et al. 2022). Two concrete tiles had 48 and 90% spat mortality within the first week of settlement and were excluded from further analysis as the remaining tiles had < 20% mortality within the first week. We also did not include tiles with <10 spat in the survivorship analysis (1 concrete tile, 18 alumina tiles, 24 CaCO3 tiles; total = 43 tiles) to minimise the influence of the initial number of settled spat on the average spat survivorship rate at the tile level. Overall, the survivorship of 6,202 individual A. kenti spat among all treatment combinations was tracked over 10 weeks.
We measured the size of spat (up to 20 random spat per tile) that survived to 10 weeks. We also assessed the role of macroalgal interactions in influencing spat survivorship by estimating the proportion of the perimeter of each spat in contact with macroalgae (0: no macroalgae, >0–≤ 0.25, 0.25–≤ 0.5, 0.5–≤ 0.75, 0.75–1). Macroalgal interactions were quantified at the time points when the spat were recorded as dead or at the final time point (i.e., week 10) if the spat were alive. All image analyses, including the tracking of the survivorship of spat, the size of spat, and the interactions between spat and macroalgae were performed in the ImageJ software (Schneider et al. 2012).
Statistical analyses
All analyses were performed in R v4.2.1 (R Core Team 2022). The percent cover of macroalgal communities were square-root transformed and visualised using non-metric multi-dimensional scaling (nMDS) based on Bray–Curtis dissimilarity. Differences in the macroalgal community composition among material types and surface roughness were examined using a permutation multivariate analysis of variance (PERMANOVA) with adonis2 function (vegan package; Oksanen et al. 2022). To account for the variation in the growing conditions among tanks and the nested experimental design, we fitted ‘mesocosm tank’ as the first term in the PERMANOVA models to absorb as much variance as possible due to the tank effect and restricted the permutations of macroalgal communities on the tiles within each tank to test the main effects of ‘material’ and ‘roughness’ (n = 9,999 permutations). Similarity percentage (SIMPER) analysis was then conducted to evaluate which macroalgal taxa contributed to the significant differences in the pairwise comparisons among treatments. Comparisons between macroalgal communities colonising the tiles and the established tank communities were only done descriptively due to strongly imbalanced number of replicates (36 quadrats of tank communities vs. 450 tiles across six tanks).
To determine whether there were differences in the settlement success of A. kenti larvae among treatments, we used generalised linear mixed-effects models (GLMMs; glmmTMB package; Brooks et al. 2017) to compare the density of settled larvae among material types and surface roughness. Both mesocosm tanks and settlement choice assay tanks were included as random effects in the models. Negative binomial distribution was used to fit the over-dispersed count data of the number of settled larvae, while the tile area was included as an offset term to express the response variable as settlement density (number of larvae per cm2). We also added ‘material’ in the zero-inflation and dispersion model formula to capture the zero-inflated data and heteroscedasticity among the material treatment.
To compare the survivorship rates of coral spat, GLMMs with binomial distribution were performed to model the proportion of spat that were alive on each tile as a function of material, roughness, and age of spat (1, 4, 7, and 10 weeks old). We included ‘mesocosm tank’ as a random effect and a temporal autocorrelation with lag 1 (AR1) to account for the repeated measurement of spat across time.
GLMMs were also used to assess whether there were differences in the size of spat and relative early recruitment success among material types and surface roughness of settlement tiles. Relative recruitment success for each tile was calculated by multiplying the larval settlement success rates by the spat survival rates. Gaussian distribution was fitted in the GLMMs to analyse the size of spat that were alive at the end of the experiment (i.e., 10-week-old spat) while Tweedie distribution was used in the relative recruitment success models. Similar to the two previous models, we added ‘mesocosm tank’ as a random effect.
Significance of fixed effects in the GLMMs were assessed using the likelihood ratio tests. Post-hoc analyses were conducted using the emmeans package with Tukey’s adjusted p-values (Lenth 2022). Assumptions on the normality and heteroscedasticity of residuals of all GLMMs were graphically validated using the DHARMa package (Hartig 2022).
Results
Macroalgal communities
Following 6 weeks of conditioning, the macroalgal communities on the tiles were significantly different among the six tanks (PERMANOVA: R2 = 0.385, Pseudo-F = 63.7, p = 0.0001). The nMDS plots showed clear separation among tanks, particularly Tank 1 and 2 being more dominated by diatoms (Fig. 3a, Fig. 4a). The gametophyte of the brown alga Colpomenia sp., which exhibits a crustose growth form, was common on tiles in Tanks 3, 5, and 6, while the crustose coralline alga Crustaphytum sp. was the most abundant in Tank 4, consistent with the patterns observed in the established communities of the mesocosm tanks (Fig. 3a, Fig. 4a). When permutations of PERMANOVA were restricted by each tank, we found significant differences in the macroalgal communities among material types (R2 = 0.08, Pseudo-F = 33.0, p = 0.0001), but not roughness levels (R2 = 0.007, Pseudo-F = 1.42, p = 0.163). Post-hoc analyses showed significant differences in all pairwise comparisons among the material types, which were mainly driven by the differences in the abundance of the CCA Lithophyllum sp. and Crustaphytum sp. (ESM Table S1).
At the end of the experiment (i.e., 10 weeks post-settlement), the differences in the macroalgal communities on the tiles among mesocosm tanks were still evident (PERMANOVA: R2 = 0.309, Pseudo-F = 35.2, p = 0.0001; Fig. 3b). Compared to tiles from other tanks, tiles from Tanks 2 and 3 had higher cover of Lithophyllum sp. and Lobophora sp., respectively (Fig. 4b). Similar patterns were observed in the tank communities, where Lithophyllum sp. and Lobophora sp. had the highest abundance in Tanks 2 and 3, respectively (Fig. 4b, ESM Fig. S4b). Within each tank, we found significant effects of materials and roughness on the macroalgal compositions (material: R2 = 0.219, Pseudo-F = 62.2, p = 0.0001; roughness: R2 = 0.0126, Pseudo-F = 3.58, p = 0.0026). Post-hoc analyses showed significant differences in all pairwise comparisons among material types, but none among surface roughness after adjustment of p-values following the Bonferroni’s method. SIMPER analysis revealed that Crustaphytum sp. was the main taxa responsible for the differences between alumina and CaCO3 tiles, while the abundance of Lithophyllum sp., Crustaphytum sp., and biofilm had the greatest contributions to the dissimilarities between CaCO3 and concrete tiles (ESM Table S2).
Larval settlement choice
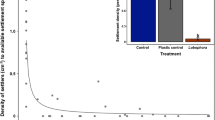
The density of A. kenti larvae that successfully settled on the settlement tiles differed significantly among materials (df = 2, χ2 = 106.0, p < 0.001), but not roughness (df = 4, χ2 = 4.41, p = 0.354; Fig. 5a). We found that settlement density was ~4.5× greater on concrete tiles (1.92 ± 0.10 larvae cm−2; mean ± SE) compared to alumina (0.43 ± 0.06 larvae cm−2) and CaCO3 tiles (0.41 ± 0.05 larvae cm−2; Fig. 5a). The settlement density on each tile also varied greatly by where the tiles were conditioned (σtank = 0.225; ESM Fig. S5a). No strong positive correlations were observed between CCA cover and settlement density (ESM Fig. S6).
The estimated marginal means and their 95% confidence intervals of a the settlement density of coral larvae, b survivorship of coral spat over 10 weeks, c size of coral spat at the end of the experiment, and d relative recruitment success (larval settlement × spat survival rates) among material types and surface roughness of settlement tiles
Spat survivorship, size, and interactions with macroalgae
The survivorship of coral spat declined significantly across all treatments over the course of 10 weeks (Fig. 5b). The rate of decline in spat survivorship did not differ among surface roughness for each material type; however, at roughness 1, the rate of decline in the spat survivorship was 31.0–34.6% greater on alumina and concrete tiles compared to CaCO3 tiles (Fig. 5b). At 10-week-old, the odds ratio of spat being alive was 4.66–7.36× greater on some of CaCO3 tile treatments compared to alumina and concrete tiles (ESM Fig. S7). An average of 73.5% (55.3–86.1%, 95% confidence intervals (CI)) of total spat survived on CaCO3 tiles, while the mean survival rates on alumina and concrete tiles were 39.0% (22.6–58.3%) and 37.8% (22.6–55.7%), respectively. The survivorship of coral spat also varied greatly by tanks (σtank = 0.802; ESM Fig. S5b).
There were significant differences in the size of 10-week-old spat among material types (df = 2, χ2 = 9.97, p = 0.007), but not surface roughness (df = 2, χ2 = 3.96, p = 0.138; Fig. 5c). In particular, spat on concrete tiles were larger than those on CaCO3 tiles, but these differences were small (Δsize = 0.096 ± 0.031 mm2; Fig. 5c). On average, spat grew from 0.615 ± 0.027 mm2 when they first settled to 0.923 ± 0.008 mm2 at 10-week-old. Both live and dead spat were most frequently observed to have 75–100% of their perimeter being in contact with macroalgae after 10 weeks (Fig. 6). However, 88.3% out of 3,400 spat that were dead had between 75–100% of their perimeter in contact with macroalgae, predominantly Crustaphytum sp. and Lobophora sp., while only 30.3% out of 2,802 spat that were alive had 75–100% direct contact with macroalgae (Fig. 6).
Relative early recruitment success
When both settlement and survivorship rates were considered to evaluate the overall early recruitment success (proportion successfully settled and survived at week 10), concrete tiles had 2.1–7.7× higher relative recruitment success compared to alumina and CaCO3 tiles across all three roughness levels (Fig. 5d, ESM Fig. S8). For each material type, there were no significant differences in the relative recruitment success among roughness level, except for alumina tiles with roughness 1 having higher recruitment success than alumina tiles with roughness 3 (ESM Fig. S8).
Discussion
Sexual propagation of corals is a promising strategy for upscaled coral restoration, but one of the main challenges is the high mortality of coral spat during the early phase of their lives. Optimising the properties of settlement substrates such as material types and surface roughness has the potential to enhance coral recruitment success by limiting competition between newly settled spat and macroalgae. The results of this study indicate that surface roughness had little impact in influencing macroalgal community assemblages on the settlement tiles and subsequent coral recruitment success. Mesocosm tanks in which the tiles were conditioned and held during the spat grow-out period (influenced by their pre-established communities) were the strongest determinant of the macroalgal compositions on the tiles, followed by material types. Settlement of A. kenti larvae was substantially higher on concrete tiles, but spat survival was higher on CaCO3 tiles. Nevertheless, when taking into account both settlement and survival rates, concrete tiles were the best performing among the tile types tested here.
Effects of material types and surface roughness on macroalgal communities
Macroalgal communities on the settlement tiles were strongly driven by mesocosm tanks (each with different established macroalgal communities), explaining 30.9–38.5% of the variance in the community structure. Macroalgal communities were also significantly different among material types, but the percentage of variance explained was relatively lower (8% following six weeks conditioning and 21.9% at week 10 post-settlement). Similar patterns were observed in the field by Burt et al. (2009), who also found that benthic communities on tiles of five different materials (concrete, gabbro, granite, sandstone, and terracotta) in the Persian Gulf were more strongly driven by sites rather than material types. Likewise, Kennedy et al. (2017) reported that total CCA abundance on settlement tiles differed significantly among habitat types in the southern Great Barrier Reef, but not among the six tile materials (polycarbonate, PVC, terracotta, limestone, porcelain, glass). The succession patterns of macroalgal assemblages are highly dynamic and dependent on a range of biotic and abiotic factors, including propagule supply, light intensity, nutrient availability, water movement, temperature, competition with other benthic organisms, and grazing by herbivores (Fletcher and Callow 1992; Keith et al. 2014; Wahl et al. 2015). In our study, we noted that the proliferation of Lithophyllum sp. and Lobophora sp. was only observed on tiles in Tanks 2 and 3, respectively, where these macroalgae had the highest cover in the established tank communities among the six tanks (Fig. 4). This suggests that the availability of macroalgal spores produced by the established macroalgal communities within each mesocosm tank was likely a critical factor in determining the abundance and composition of macroalgae on the settlement tiles. In addition, established CCA communities in the tanks might produce biochemicals that suppressed growth and recruitment of other macroalgae (Suzuki et al. 1998; Kim et al. 2004; Vermeij et al. 2011; Gomez-Lemos and Diaz-Pulido 2017), promoting CCA abundance on the settlement tiles. Nevertheless, it should be noted that the patterns of macroalgal communities colonising the tiles were highly influenced by the established macroalgal communities in the mesocosm tanks, which were CCA-dominated, potentially affecting the generalisability of our findings to other tank and reef systems characterised by more diverse macroalgal species.
Modifying the surface roughness of the settlement tiles had very limited effects on macroalgal community compositions. Surface roughness has been reported in several studies to influence the settlement rates of fouling organisms, including macroalgae (Callow et al. 2002; Scardino et al. 2007; Schumacher et al. 2007). The lack of influence of surface roughness on the macroalgal communities in this study may have been due to the dominance of CCA that produce non-motile spores in the tanks (i.e., Crustaphytum sp. and Lithophyllum sp.; ESM Table S3). Scardino et al. (2007) reported that surface microtopography influenced the fouling rates of organisms with motile propagules such as the green alga Ulva rigida, the tube worm Hydroides elegans and the bryozoan Bugula neritina, but not those with non-motile propagules such as the red alga Centroceras clavulatum. Using silica beads as controls for non-motile spores, Callow et al. (2002) found that the settlement rates of beads were similar across various topographies, while the spores of the green alga Ulva linza preferentially settled on the substratum with microtopography that matched their body size (5 µm). As non-motile macroalgal spores would settle passively on the substratum without performing surface exploration, the settlement rates would be equal across all surfaces, unless settled spores had weak adhesion strength and were dislodged from the substratum (Scardino et al. 2008). Once settled, it was unlikely that Crustaphytum sp. and Lithophyllum sp. germlings detached from the tiles as the calcification process in CCA occurs within hours after settlement (de Carvalho et al. 2022).
Our findings further emphasize the importance of understanding existing macroalgal communities and the characteristics of reproductive propagules that are present (e.g., size, motility) in designing suitable settlement substrates. Modifying surface roughness will likely have a limited impact on controlling the fouling of macroalgae if the benthic communities are dominated by red macroalgae, which generally produce non-motile spores. Another important consideration is the spatial scale of roughness relative to the size of the macroalgal spores as the attachment points between settling macroalgal spores and substratum are the weakest when the surface roughness of the substratum is slightly smaller than the width of the macroalgal spores (Callow et al. 2002; Schumacher et al. 2007; Scardino et al. 2008). However, macroalgae are highly diverse and the size of their reproductive cells ranges from 2 µm to 250 µm (Clayton 1992). In this study, the average height profile (Sa) ranged from 1.6 µm on Roughness 1 tiles to 16.0 µm on Roughness 5 tiles, while the maximum valley depth (Sv) was between 42.8 and 116.2 µm. An effective deterrent surface may therefore require hierarchical patterning of roughness in multiple length scales (Callow and Callow 2011).
Effects of material types and surface roughness on early coral recruitment success
Significantly higher numbers of A. kenti larvae preferred to settle on concrete tiles than alumina and CaCO3 tiles. This was potentially related to the abundance of CCA on the tiles as many studies have shown that CCA can induce settlement in coral larvae (Heyward and Negri 1999; Diaz-Pulido et al. 2010; Tebben et al. 2015; Abdul Wahab et al. 2023). In this study, while we observed that concrete tiles had higher percent cover of Crustaphytum sp. and Lithophyllum sp. compared to alumina and CaCO3 tiles, CCA cover was not strongly positively correlated with settlement success (ESM Fig. S6). Lobophora sp., belonging to a genus of brown macroalgae that is known to inhibit larval settlement (Evensen et al. 2019; Fong et al 2019; but see Birrell et al. 2008b), had very low cover (average of 1.3–4.4%) across the tile types during the larval settlement assays. It remains unclear which innate (chemical or physical) properties of concrete might have also played a role in attracting coral larvae. Settlement success was low on CaCO3 tiles, which was unexpected given that several studies have noted high settlement of coral larvae on dead coral skeleton and coral rubble, also comprised of CaCO3 (Heyward and Negri 1999; Ritson-Williams et al. 2010). However, the CaCO3 tiles used in this study were made from the compaction of CaCO3 powder, unlike dead coral skeleton and rubbles, which possess complex pore structures that can potentially serve as a physical cue for larval settlement (Whalan et al. 2015) and residual biochemicals that may include settlement cues (Heyward and Negri 1999).
The survivorship of A. kenti spat declined significantly over the course of 10 weeks across all treatments. Among the 3,400 dead spat, 88.3% had 75–100% of their perimeter in contact with macroalgae, predominantly Crustaphytum sp. and Lobophora sp. Notably, we observed that Crustaphytum sp. and Lobophora sp. often grew over the spat and killed them (i.e., active overgrowth) within seven weeks from first contact. Despite the low light environment (maximum midday irradiance of 50 PAR), Crustaphytum sp. and Lobophora sp. grew rapidly and occupied most of the available space on the settlement tiles within 10 weeks post-settlement, outcompeting the coral spat before they reached an escape threshold (i.e., large enough to resist the overgrowth). Importantly, our results demonstrate that overgrowth by CCA can be an important mechanism that mediates the interactions between benthic macroalgae and corals. Empirical evidence of space competition between CCA and coral is scarce as most studies focused on the influence of CCA on coral larval settlement preference (Heyward and Negri 1999; Abdul Wahab et al. 2023). Early work by Harrington et al. (2004) noted the overgrowth of A. tenuis spat by Porolithon onkodes and Titanoderma prototypum, but concluded that overgrowth played a minor role in mediating the competition between CCA and spat. They found that a more effective strategy employed by CCA in preventing the survival of coral spat was by epithallial sloughing (e.g., in Neogoniolithon fosliei, P. onkodes, and Hydrolithon reinboldii). A recent study by Jorissen et al. (2020) showed that CCA species that typically grew in sub-cryptic habitats (N. fosliei and Paragoniolithon conicum) were competitively dominant against Pocillopora sp. recruits and frequently killed the recruits via overgrowth, while the two CCA species that were commonly found in exposed habitats (P. onkodes and Lithophyllum insipidum) helped to promote the survival of Pocillopora sp. recruits. CCA may also possess allelochemicals that are potentially harmful to coral spat. Previous studies have demonstrated the allelopathic activities of some CCA extracts in inhibiting the settlement of coral larvae (Harrington et al. 2004) and other macroalgal spores (Suzuki et al. 1998; Kim et al. 2004; Vermeij et al. 2011; Gomez-Lemos and Diaz-Pulido 2017). Different CCA species likely have different competitive abilities against coral spat depending on their sloughing ability, allelopathic potency, growth rate, size, crust thickness, and environmental conditions (Harrington et al. 2004; Jorissen et al. 2020; Abdul Wahab et al. 2023). Further studies are required to better understand the variation in the competitive abilities of CCA species against newly settled corals and how it may relate to their facilitative effects in inducing coral larval settlement.
Spat mortality was lower on CaCO3 tiles compared to alumina and concrete tiles, particularly at Roughness 5. The mean survival of 10-week-old A. kenti spat on CaCO3 tiles was 73.5%, while only 37.8 and 39% of total spat were alive on alumina and concrete tiles, respectively. This was likely driven by less intense space competition on CaCO3 tiles, which had 53.2 ± 2.5% cover of biofilm, or ‘bare space’, available for benthic macroorganisms to colonize. In contrast, the biofilm cover on alumina and concrete tiles was 18.5 ± 1.6% and 12.7 ± 1.5%, respectively. Nevertheless, despite the low post-settlement survival, concrete tiles had the highest overall early recruitment success among the material types. Successful recruitment is dependent on corals passing through all early life-history stages, from fertilisation to settlement to post-settlement survival (Birrell et al. 2008a). While spat survival was higher on CaCO3 than concrete tiles, CaCO3 tiles performed considerably worse during the settlement process. The number of coral larvae that successfully settled and metamorphosed on CaCO3 tiles was 0–134 larvae, with 13 out of 54 tiles having zero settlement. In contrast, concrete tiles had 8–259 settled larvae. Consequently, the overall recruitment success on CaCO3 tiles was low.
Conclusion
Overall, concrete was found to be the best performing settlement substrate with the highest early recruitment success (up to 10-week-old) among the three materials tested in this study. The mesocosm tanks were the strongest determinant of the macroalgal community structures on the tiles, overwhelming the effects of material types. Surface roughness did not influence the macroalgal community assemblages on the settlement tiles; consequently, modifying surface roughness did not improve the settlement success of coral larvae and their early post-settlement survival. Very strong competitive interactions were observed between coral spat and macroalgae, with overgrowth by CCA being the primary cause of high spat mortality in the aquaculture system over the first 10 weeks post-settlement. Loss of coral spat to overgrowth by CCA represents a potential bottleneck in scaling up the restoration efforts to seed sexually propagated corals. However, identification of this important mechanism informs opportunities to optimise early spat survival until deployment, including further investigation of material types, reducing light levels, and introducing juvenile sea urchins (e.g., Echinometra mathaei and Tripneustes gratilla) to control CCA as well as filamentous and fleshy macroalgal abundance (Neil et al. 2024). Greater labour investment in husbandry during the early life stages may also be necessary to overcome the bottlenecks of post-settlement mortality (Edwards et al. 2015). Another option is to culture and promote the dominance of less competitively aggressive CCA on the settlement tiles; for example, Titanoderma spp. have very thin crusts and likely pose low risk of overgrowing coral spat (Ritson-Williams et al. 2010). Importantly, while this study sought to optimise the properties of settlement substrates to improve coral settlement and early post-settlement survival for coral seeding, additional work is required to determine the longer-term survival and growth of the coral spat following their deployment onto reefs to assess the effectiveness of this restoration strategy.
References
Abdul Wahab MA, Ferguson S, Snekkevik VK, McCutchan G, Jeong S, Severati A, Randall CJ, Negri AP, Diaz-Pulido G (2023) Hierarchical settlement behaviours of coral larvae to common coralline algae. Sci Rep 13:5795
Anderson MJ (1996) A chemical cue induces settlement of Sydney rock oysters, Saccostrea commercialis, in the laboratory and in the field. Biol Bull 190:350–358
Antink MM, Roepke L, Bartels J, Soltmann C, Kunzmann A, Rezwan K, Kroll S (2018) Porous ceramics with tailored pore size and morphology as substrates for coral larval settlement. Ceram Int 44:16561–16571
Babcock R, Smith L (2002) Effects of sedimentation on coral settlement and survivorship. Proc 9th Int Coral Reef Symp 245–248.
Banaszak AT, Marhaver KL, Miller MW, Hartmann AC, Albright R, Hagedorn M, Harrison PL, Latijnhouwers KR, Mendoza Quiroz S, Pizarro V, Chamberland VF (2023) Applying coral breeding to reef restoration: best practices, knowledge gaps, and priority actions in a rapidly evolving field. Restor Ecol 31:e13913
Baums IB, Baker AC, Davies SW, Grottoli AG, Kenkel CD, Kitchen SA, Kuffner IB, LaJeunesse TC, Matz MV, Miller MW, Parkinson JE, Shantz AA (2019) Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol Appl 29:e01978
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA (2008a) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Annu Rev 46:25–63
Birrell CL, McCook LJ, Willis BL, Harrington L (2008b) Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar Ecol Progr Ser 362:129–137
Boström-Einarsson L, Babcock RC, Bayraktarov E, Ceccarelli D, Cook N, Ferse SC, Hancock B, Harrison P, Hein M, Shaver E, Smith A, Suggett D, Steward-Sinclair PJ, Cardi T, McLeod I (2020) Coral restoration— a systematic review of current methods, successes, failures and future directions. PLoS ONE 15:e0226631
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Bridge TC, Cowman PF, Quattrini AM, Bonito VE, Sinniger F, Harii S, Head CE, Hung JY, Halafihi T, Rongo T, Baird AH (2023) A tenuis relationship: traditional taxonomy obscures systematics and biogeography of the ‘Acropora tenuis’ (Scleractinia: Acroporidae) species complex. Zoo J Linn Soc : zlad062.
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9:378–400
Bulleri F, Thiault L, Mills SC, Nugues MM, Eckert EM, Corno G, Claudet J (2018) Erect macroalgae influence epilithic bacterial assemblages and reduce coral recruitment. Mar Ecol Prog Ser 597:65–77
Burt J, Bartholomew A, Bauman A, Saif A, Sale PF (2009) Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. J Exp Mar Biol Ecol 373:72–78
Butler CC, Turnham KE, Lewis AM, Nitschke MR, Warner ME, Kemp DW, Hoegh-Guldberg O, Fitt WK, van Oppen MJ, LaJeunesse TC (2023) Formal recognition of host-generalist species of dinoflagellate (Cladocopium, Symbiodiniaceae) mutualistic with Indo-Pacific reef corals. J Phycol 59:698–711
Callow JA, Callow ME (2011) Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat Commun 2:244
Callow ME, Jennings AR, Brennan AB, Seegert CE, Gibson A, Wilson L, Feinberg A, Baney R, Callow JA (2002) Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling 18:229–236
Chamberland VF, Petersen D, Guest JR, Petersen U, Brittsan M, Vermeij MJ (2017) New seeding approach reduces costs and time to outplant sexually propagated corals for reef restoration. Sci Rep 7:18076
Clayton MN (1992) Propagules of marine macroalgae: structure and development. Br Phycol J 27:219–232
De Carvalho RT, Wendt CH, Willemes MJ, Bahia RD, Farina M, Salgado LT (2022) Ontogeny and early steps of the calcification process in coralline algae Lithophyllum corallinae (Florideophyceae, Rhodophyta). Front Mar Sci 9:900607
Diaz-Pulido G, McCook LJ (2004) Effects of live coral, epilithic algal communities and substrate type on algal recruitment. Coral Reefs 23:225–233
Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O (2010) The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 29:203–208
Doropoulos C, Roff G, Bozec YM, Zupan M, Werminghausen J, Mumby PJ (2016) Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr 86:20–44
Edwards AJ, Guest JR, Heyward AJ, Villanueva RD, Baria MV, Bollozos IS, Golbuu Y (2015) Direct seeding of mass-cultured coral larvae is not an effective option for reef rehabilitation. Mar Ecol Prog Ser 525:105–116
Evensen NR, Doropoulos C, Morrow KM, Motti CA, Mumby PJ (2019) Inhibition of coral settlement at multiple spatial scales by a pervasive algal competitor. Mar Ecol Prog Ser 612:29–42
Field SN, Glassom D, Bythell J (2007) Effects of artificial settlement plate materials and methods of deployment on the sessile epibenthic community development in a tropical environment. Coral Reefs 26:279–289
Fletcher RL, Callow ME (1992) The settlement, attachment and establishment of marine algal spores. Br Phycol J 27:303–329
Fong J, Lim ZW, Bauman AG, Valiyaveettil S, Liao LM, Yip ZT, Todd PA (2019) Allelopathic effects of macroalgae on Pocillopora acuta coral larvae. Mar Environ Res 151:104745
Gomez-Lemos LA, Diaz-Pulido G (2017) Crustose coralline algae and associated microbial biofilms deter seaweed settlement on coral reefs. Coral Reefs 36:453–462
Hartig F (2022). DHARMa: Residual Diagnostics for Hierarchical (Multi–Level / Mixed) Regression Models. R package version 0.4.5. https://CRAN.R-project.org/package=DHARMa.
Hauri C, Fabricius KE, Schaffelke B, Humphrey C (2010) Chemical and physical environmental conditions underneath mat- and canopy-forming macroalgae, and their effects on understorey corals. PLoS ONE 5:e12685
Heyward AJ, Negri AP (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279
Huisman J (2015) Algae of Australia: marine benthic algae of north–western Australia, 1. Australian Biological Resources Study & CSIRO Publishing, Canberra & Melbourne, Green and brown algae
Jorissen H, Baumgartner C, Steneck RS, Nugues MM (2020) Contrasting effects of crustose coralline algae from exposed and subcryptic habitats on coral recruits. Coral Reefs 39:1767–1778
Keith SA, Kerswell AP, Connolly SR (2014) Global diversity of marine macroalgae: environmental conditions explain less variation in the tropics. Glob Ecol Biogeogr 23:517–529
Kennedy EV, Ordoñez A, Lewis BE, Diaz-Pulido G (2017) Comparison of recruitment tile materials for monitoring coralline algae responses to a changing climate. Mar Ecol Prog Ser 569:129–144
Kim MJ, Choi JS, Kang SE, Cho JY, Jin HJ, Chun BS, Hong YK (2004) Multiple allelopathic activity of the crustose coralline alga Lithophyllum yessoense against settlement and germination of seaweed spores. J Appl Phycol 16:175–179
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Kraft GT (2007) Algae of Australia: marine benthic algae of Lord Howe Island and the Southern Great Barrier Reef, 1. Australian Biological Resources Study & CSIRO Publishing, Canberra & Melbourne, Green Algae
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Lenth R (2022). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.7.5. https://CRAN.R-project.org/package=emmeans
Leonard C, Hédouin L, Lacorne MC, Dalle J, Lapinski M, Blanc P, Nugues MM (2022) Performance of innovative materials as recruitment substrates for coral restoration. Restor Ecol 30:e13625
Levenstein MA, Marhaver KL, Quinlan ZA, Tholen HM, Tichy L, Yus J, Lightcap I, Wegley Kelly L, Juarez G, Vermeij MJ, Wagoner Johnson AJ (2022) Composite substrates reveal inorganic material cues for coral larval settlement. ACS Sustain Chem Eng 10:3960–3971
Ligson CA, Cabaitan PC, Harrison PL (2022) Survival and growth of coral recruits in varying group sizes. J Exp Mar Biol Ecol 556:151793
Neil RC, Humphrey C, Bourne DG, Heyward A (2021) Co-culture with grazers can improve survival and growth of multiple coral species. Aquaculture 544:737095
Neil RC, Barton JA, Dougan W, Dworjanyn S, Heyward A, Mos B, Bourne DG, Humphrey C (2024) Size matters: microherbivores make a big impact in coral aquaculture. Aquaculture 581:740402
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) Vegan: community ecology package. R Package Version 2:6–2
Paul VJ, Kuffner IB, Walters LJ, Ritson-Williams R, Beach KS, Becerro MA (2011) Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser 426:161–170
Penin L, Michonneau F, Carroll A, Adjeroud M (2011) Effects of predators and grazers exclusion on early post-settlement coral mortality. Hydrobiologia 663:259–264
Petersen D, Laterveer M, Schuhmacher H (2005a) Innovative substrate tiles to spatially control larval settlement in coral culture. Mar Biol 146:937–342
Petersen D, Laterveer M, Schuhmacher H (2005b) Spatial and temporal variation in larval settlement of reefbuilding corals in mariculture. Aquaculture 249:317–327
Price IR, Scott FJ (1992) The turf algal Flora of the Great Barrier Reef: Part I Rhodophyta. James Cook University of North Queensland, Townsville, Botany Department
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Randall CJ, Giuliano C, Heyward AJ, Negri AP (2021) Enhancing coral survival on deployment devices with microrefugia. Front Mar Sci 8:662263
Randall CJ, Giuliano C, Allen K, Bickel A, Miller M, Negri AP (2023) Site mediates performance in a coral-seeding trial. Restor Ecol 31:e13745
Randall CJ, Negri AP, Quigley KM, Foster T, Ricardo GF, Webster NS, Bay LK, Harrison PL, Babcock RC, Heyward AJ (2020) Sexual production of corals for reef restoration in the Anthropocene. Mar Ecol Prog Ser 635:203–232
Ritson-Williams R, Paul VJ, Arnold SN, Steneck RS (2010) Larval settlement preferences and post–settlement survival of the threatened Caribbean corals Acropora palmata and A. cervicornis. Coral Reefs 29:71–81
River GF, Edmunds PJ (2001) Mechanisms of interaction between macroalgae and scleractinians on a coral reef in Jamaica. J Exp Mar Biol Ecol 26:159–172
Scardino AJ, Guenther J, De Nys R (2008) Attachment point theory revisited: the fouling response to a microtextured matrix. Biofouling 24:45–53
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schumacher JF, Carman ML, Estes TG, Feinberg AW, Wilson LH, Callow ME, Callow JA, Finlay JA, Brennan AB (2007) Engineered antifouling microtopographies–effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 23:55–62
Spieler RE, Gilliam DS, Sherman RL (2001) Artificial substrate and coral reef restoration: what do we need to know to know what we need. Bull Mar Sci 69:1013–1030
Standards Australia (2016) AS 2350.12–2006 Methods of testing Portland, blended and masonry cements, Method 12: Preparation of a standard mortar and moulding of specimens, Standards Australia, Australia
Suzuki Y, Takabayashi T, Kawaguchi T, Matsunaga K (1998) Isolation of an allelopathic substance from the crustose coralline algae, Lithophyllum spp., and its effect on the brown alga, Laminaria religiosa Miyabe (Phaeophyta). J Exp Mar Biol Ecol 225:69–77
Tebben J, Motti CA, Siboni N, Tapiolas DM, Negri AP, Schupp PJ, Kitamura M, Hatta M, Steinberg PD, Harder T (2015) Chemical mediation of coral larval settlement by crustose coralline algae. Sci Rep 5:10803
Van Oppen MJ, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Cormick C, Crean A, Damjanovic K, Epstein H, Harrison PL, Jones TA, Miller M, Pears RJ, Peplow LM, Raftos DA, Schaffelke B, Stewart K, Torda G, Wachenfeld D, Weeks AR, Putnam HM (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Chang Biol 23:3437–3448
Vermeij MJ (2006) Early life-history dynamics of Caribbean coral species on artificial substratum: the importance of competition, growth and variation in life-history strategy. Coral Reefs 25:59–71
Vermeij MJ, Sandin SA (2008) Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology 89:1994–2004
Vermeij MJ, Dailer ML, Smith CM (2011) Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar Ecol Prog Ser 422:1–7
Vieira C, Thomas OP, Culioli G, Genta-Jouve G, Houlbreque F, Gaubert J, De Clerck O, Payri CE (2016) Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci Rep 6:18637
Wahl M, Molis M, Hobday A, Dudgeon SR, Neumann R, Steinberg P, Campbell AH, Marzinelli E, Connell SD (2015) The responses of brown macroalgae to environmental change from local to global scales: direct and ecologically mediated effects. Perspect Phycol 2:11–30
Wangpraseurt D, Weber M, Røy H, Polerecky L, de Beer D, Suharsono NMM (2012) In situ oxygen dynamics in coral–algal interactions. PLoS ONE 7:e31192
Whalan S, Abdul Wahab MA, Sprungala S, Poole AJ, de Nys R (2015) Larval settlement: the role of surface topography for sessile coral reef invertebrates. PLoS ONE 10:e0117675
Xiao L, Finlay JA, Röhrig M, Mieszkin S, Worgull M, Hölscher H, Callow JA, Callow ME, Grunze M, Rosenhahn A (2018) Topographic cues guide the attachment of diatom cells and algal zoospores. Biofouling 34:86–97
Acknowledgements
We thank Ateeq Rehman for his assistance with the experiment. The coral colonies used in this study were collected from the traditional sea Country of the Manbarra peoples and used in this study with their Free Prior and Informed Consent. The authors are grateful for the granting of consent and we pay our respects to Elders past, present and emerging. This research was supported by the Reef Restoration and Adaptation Program, which aims to develop effective interventions to help the Reef resist, adapt and recover from the impacts of climate change, and which is funded by the partnership between the Australian Government’s Reef Trust and the Great Barrier Reef Foundation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fong, J., Ramsby, B.D., Flores, F. et al. Effects of material type and surface roughness of settlement tiles on macroalgal colonisation and early coral recruitment success. Coral Reefs 43, 1083–1096 (2024). https://doi.org/10.1007/s00338-024-02526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02526-4