Abstract
Global warming is causing an unprecedented loss of species and habitats worldwide. This is particularly apparent for tropical coral reefs, with an increasing number of reefs experiencing mass bleaching and mortality on an annual basis. As such, there is a growing need for a standardized experimental approach to rapidly assess the thermal limits of corals and predict the survival of coral species across reefs and regions. Using a portable experimental system, the Coral Bleaching Automated Stress System (CBASS), we conducted standardized 18 h acute thermal stress assays to quantitively determine the upper thermal limits of four coral species across the length of the Red Sea coastline, from the Gulf of Aqaba (GoA) to Djibouti (~ 2100 km). We measured dark-acclimated photosynthetic efficiency (Fv/Fm), algal symbiont density, chlorophyll a, and visual bleaching intensity following heat stress. Fv/Fm was the most precise response variable assessed, advancing the Fv/Fm effective dose 50 (ED50, i.e., the temperature at which 50% of the initial Fv/Fm is measured) as an empirically derived proxy for thermal tolerance. ED50 thermal thresholds from the central/southern Red Sea and Djibouti populations were consistently higher for Acropora hemprichii, Pocillopora verrucosa, and Stylophora pistillata (0.1–1.8 °C above GoA corals, respectively), in line with prevailing warmer maximum monthly means (MMMs), though were lower than GoA corals relative to site MMMs (1.5–3.0 °C). P. verrucosa had the lowest thresholds overall. Despite coming from the hottest site, thresholds were lowest for Porites lobata in the southern Red Sea, suggesting long-term physiological damage or ongoing recovery from a severe, prior bleaching event. Altogether, the CBASS resolved historical, taxonomic, and possibly recent environmental drivers of variation in coral thermal thresholds, highlighting the potential for a standardized, short-term thermal assay as a universal approach for assessing ecological and evolutionary variation in the upper thermal limits of corals.
Similar content being viewed by others
Introduction
Global warming is impacting natural ecosystems globally, with tropical organisms living close to their thermal limits considered most at risk (Vinagre et al. 2016). This has been extensively documented for tropical coral reefs, which have been heavily impacted by pantropical thermal anomalies in recent decades (Hughes et al. 2018a; Lough et al. 2018; Skirving et al. 2019). Indeed, warming ocean temperatures are leading to increasingly frequent and intense bleaching events (Kleypas et al. 2021, Hughes et al. 2018a, 2019), which have resulted in unprecedented rates of mortality for reef-building corals (Hughes et al. 2018b). In turn, with ocean temperatures expected to further increase in the coming decades, projections indicate that > 75% of the world’s coral reefs are expected to experience annual severe bleaching before the end of the current century, even under an intermediate scenario of carbon emissions (RCP4.5; Hooidonk et al. 2016).
Despite extensive coral loss in most ocean basins, certain coral populations have emerged as capable of withstanding and/or adapting to increasing seawater temperatures (Howells et al. 2016). Most notably, corals from highly variable thermal environments in locations such as American Samoa (Oliver and Palumbi 2011; Morikawa and Palumbi 2019), the Kimberleys in northern Australia (Schoepf et al. 2015), and the central Red Sea (Pineda et al. 2013; Voolstra et al. 2020) commonly display higher thermal thresholds than nearby populations from more thermally stable environments. Yet, broadening comparisons between and beyond these regions remains challenging, due to the differences in experimental approaches employed (McLachlan et al. 2020) and the location of many reefs in remote settings far from a major research laboratory/experimental aquarium system. Thus, a standardized and portable approach is required to accurately compare thermal thresholds across studies and reefs (Grottoli et al. 2021), and identify naturally more tolerant populations both for conservation purposes and to investigate the drivers and mechanisms underpinning the ability of these corals to withstand elevated temperatures (Barshis et al. 2013; Dixon et al. 2015; Camp et al. 2018).
Here, we determine the ecological validity of the recently-developed Coral Bleaching Automated Stress System (CBASS) (Voolstra et al. 2020; Evensen et al. 2021), in conjunction with effective dose 50 (ED50)-based thermal thresholds, as a standardized approach to determine the thermal limits of corals by conducting identical short-term heat stress assays across a large spatial scale. Specifically, we assessed the thermal thresholds of four common coral species across six reef sites along a natural latitudinal thermal gradient spanning the entire length of the Red Sea, from the Gulf of Aqaba (GoA) to the Gulf of Aden outside the southern entrance to the Red Sea (~ 2100 km overwater distance; Fig. 1). Numerous studies have investigated the thermal tolerance of corals in the region, identifying thermally tolerant coral populations in the northern Red Sea and GoA (Fine et al. 2013; Grottoli et al. 2017; Osman et al. 2018), as well as populations being negatively impacted by warming sea surface temperatures in the central Red Sea (Cantin et al. 2010). However, assessments of the thermal limits for multiple coral species across a scale as large as the Red Sea have yet to be attempted within a single study, precluding the ability for direct comparisons of thermal thresholds among sites and coral species. We empirically test multiple hypotheses relating to the species-specific effects of latitude (i.e., historical climate) and recent thermal histories (e.g., bleaching events) on coral thermal tolerance. We hypothesize that (1) ED50-based thermal thresholds will be higher in corals from warmer sites/latitudes (Jokiel and Brown 2004), (2) corals from the Gulf of Aqaba will display exceptional thermal tolerance relative to their environment (Fine et al. 2013; Osman et al. 2018), and (3) thermal thresholds will differ between species at each site due to differences in traits and life-history strategies (Loya et al. 2001; van Woesik et al. 2011).
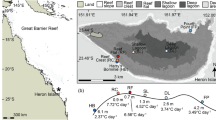
Map of the Red Sea and Gulf of Aden showing the research sites and their respective maximum monthly mean (MMM) sea surface temperatures. Temperatures are based on the NOAA Coral Reef Watch climatology data (Liu et al. 2014). Regions of the study area are indicated in italics, with Al Wajh, Yanbu, and Al Fahal all located within the Central Red Sea. Specific GPS coordinates for each site are in Table S1
Methods
Study sites and sample collection
In August 2018, replicate fragments from seven individual adult colonies of Stylophora pistillata, Porites lobata, Acropora hemprichii, and Pocillopora verrucosa were collected from five sites spanning the northern Gulf of Aqaba (29°30′N) to the southern Red Sea (18°59′N). Additionally, corals were sampled from a reef near Obock, Djibouti (12°43′N), at the edge of the Indian Ocean and mouth of the Red Sea in February 2020 (Fig. 1; Table S1). The site selection reflects a natural latitudinal gradient of seawater temperatures (Berumen et al. 2019), based on satellite measurements of the daily sea surface temperature taken by the National Oceanic and Atmospheric Administration (NOAA) since 1985 (henceforth referred to as ‘NOAA climatology’; Liu et al. 2014). NOAA climatology indicates a consistent North to South increase in maximum monthly mean (MMM) sea surface temperature within the Red Sea, before decreasing again upon reaching the southernmost Gulf of Aden (Fig. 1). There was one local-scale exception to the pattern of increasing temperature, however, with the second northernmost site, Al Wajh, displaying a slightly higher MMM (30.0 °C) than the next site further South, Yanbu (29.7 °C), likely due to increased water residence time in a nearby shallow lagoon (Fig. 1, inset). Additional details of the sample collection at each site and experimental logistics are in the supplementary material.
Short-term heat stress assays (CBASS)
We used the Coral Bleaching Automated Stress System (CBASS) to conduct identical, short-term heat stress assays (Voolstra et al. 2020; Evensen et al. 2021). The system consisted of eight 10-L flow-through tanks that allowed running four independent temperature profiles and two replicate tanks per temperature treatment (4 temperatures × 2 replicated tanks). Full technical details of the CBASS are in the supplementary material. CBASS acute thermal stress assays were designed to capture the full biological stress response of corals, with temperature profiles spanning little to no heat stress (30 °C baseline temperature, based on average maximum monthly mean temperatures [MMM] across sites) and mild (33 °C), moderate (36 °C), and extreme (39 °C) heat stress. Collected coral fragments from all seven colonies of each species at each location were distributed over eight flow-through tanks, which included two replicate tanks per temperature treatment. Thus, fragments from each colony were in each treatment condition and replicate tank to make a total of 56 fragments/species per site (7 colonies × 4 temperatures × 2 replicate tanks). For most sites, two species were run the first day at a particular site and the other two species were run the following day (56 fragments × 2 species = 112 fragments/experiment). Fragments (micro-cores for P. lobata and branch tips for the other species) were positioned upright in the tanks and secured in place using plastic grids (‘egg crate’) at the bottom of the tanks. The experiments started at 13:00 h at a control temperature of 30 °C. The control tank remained at 30 °C and the treatment tanks were heated to 33 °C, 36 °C, and 39 °C over a period of 3 h (13:00–16:00), held for 3 h at the maximum temperature (16:00–19:00), and then decreased back to 30 °C over the course of 1 h (19:00–20:00). All corals were kept at 30 °C overnight until sampling the following morning (07:00), completing the 18 h short-term heat stress assay (Fig. S1). Temperature profiles were successfully controlled across experiments, with hold temperatures averaging 30.5 °C, 33.2 °C, 36.0 °C, and 39.1 °C across all experiments for the targeted hold temperatures of 30 °C, 33 °C, 36 °C , and 39 °C, respectively (full summary of hold temperatures in Table S2).
Physiological measurements
For measurements of dark-acclimated photosynthetic efficiency, experimental tanks were covered with a tarp at 19:00 to ensure complete darkness for at least 45 min before measuring dark-acclimated photosynthetic efficiency (Fv/Fm) of photosystem II (PSII) on all coral fragments. Fv/Fm measurements were conducted using a diving PAM (Pulse Amplitude Modulated; Walz, Effeltrich, Germany) fluorometer in Al Wajh, Yanbu, Al Fahal, and Al Qunfudhah (settings: MI: 3, Gain: 6, DAMP: 2, SI: 8, SW: 0.8 s) and an imaging PAM in Eilat and Obock (settings: MI: 1, Gain: 2, DAMP: 2, SI: 10, SW: 0.8 s). Settings for both PAM instruments were adjusted prior to the experimental measurements to produce initial fluorescence readings of ~ 200–500 Ft, as per the manufacturer’s recommendations. Fv/Fm describes the maximum potential capacity of PSII to capture light energy for photosynthesis if all capable reaction centers were open, which can serve as an indicator of algal symbiont performance in response to temperature changes (Schoepf et al. 2015; Jurriaans and Hoogenboom 2019; Evensen et al. 2021) and severity of coral bleaching (Warner et al. 1999).
At the end of the experiments, all coral fragments were photographed (Nikon 1 J1 with a 10-30 mm VR Lens, Tokyo, Japan) with a monochromatic grayscale reference (Kodak grayscale), before being snap-frozen in liquid nitrogen and stored at − 80 °C until further processing. Pictures were color-corrected (sensu Winters et al. 2009) and used to conduct visual bleaching assessments (sensu Morikawa and Palumbi 2019), with fragments classified in one of six categories: 0% bleached, 20% bleached, 40% bleached, 60% bleached, 80% bleached, or 100% bleached. Categories represent the extent of bleaching across the entire fragment (Fig. S2). Three people scored bleaching independently for each fragment and the median category was used for the statistical analysis.
For the destructive physiological measurements, all coral tissue was sprayed off from the frozen corals using airflow from a sterile 1000 µl pipet tip connected via a rubber hose to a benchtop air pressure valve and ice-cold 0.2 µm filtered seawater (FSW). Subsequently, tissue slurry was homogenized using a MicroDisTec homogenizer 125 (Thermo Fisher Scientific, Waltham, MA, USA) with 200 µl aliquoted for Symbiodiniaceae cell counts (LaJeunesse et al. 2018) and 100 µl aliquoted for chlorophyll (chl) concentrations each stored at – 20 °C. Details of symbiont density and chl a measurements are in the supplementary material. Remaining coral skeletons were retained for surface area determination using the paraffin wax dipping method of Stimson and Kinzie (1991) as modified by Holmes et al. (2008), with physiological measurements then normalized to coral surface area (cm2).
Statistical analyses
All statistical analyses and data plotting were conducted in R v. 3.6.1 (R Core Team 2018), with figures plotted using ‘ggplot2’ (Wickham 2016). In order to quantitatively compare thermal thresholds between coral populations, measurements of Fv/Fm and visual bleaching assessments were fitted with log-logistic dose–response curves (DRCs) using the package ‘drc’ (Ritz et al. 2015), with DRCs fit for each individual colony based on both replicates (using the three-parameter log-logistic model; LL.3). This approach incorporated both variances due to the experimental approach and biological variance from the responses of individual colonies from each site. From these, the ED50 parameter (effective dose 50) was obtained for each colony, representing the x-values at the inflection point of the curve (in this case the temperature) where Fv/Fm or visual bleaching in the model fit was 50% lower than the starting values. Importantly, individual ED50s were calculated for each colony at each site, allowing for statistical comparison of thermal thresholds among sites that included within-species and within-site biological variability. In turn, maximum monthly mean (MMM) sea surface temperatures for each site were obtained from custom queries of the 5 km NOAA Coral Reef Watch database (Liu et al. 2014) and subtracted from the thermal thresholds (i.e., ED50s) to produce a thermal threshold relative to local conditions. Both absolute and relative thresholds were compared among sites for each species individually, as not all sites contained all species, using one-way ANOVAs. Additionally, relative thresholds were compared among sites using a generalized linear mixed-effects model (GLMM), with species included as a random effect.
Measurements of algal symbiont density and chl a across the temperature treatments were compared among sites for each species using GLMMs to evaluate differences in degree of bleaching. Temperature and site were included as fixed effects, with genotype nested within site and replicate nested within temperature included as random effects. Due to issues with temperature control during the overnight portion of certain experimental assays or a lack of analytical equipment, measurements of symbiont density and chl a were not conducted for S. pistillata and P. lobata in Al Wajh (technical issues), A. hemprichii and P. verrucosa in Al Fahal (technical issues), and the three species sampled in Obock (lack of analytical equipment). Issues with the overnight control did not affect Fv/Fm measurements as these were conducted before the overnight hold, thus only symbiont density and chl a measurements were omitted for those experiments. GLMMs were performed using the ‘aov’ function in R (R Core Team 2018) and posthoc comparisons were conducted using ‘emmeans’ (Lenth et al. 2020). For all models, distributions of the residuals were plotted to ensure normality, and residuals were plotted against fitted values to confirm homoscedasticity. Alternatively, data were log-transformed to meet these assumptions.
Results
Using the Fv /F m ED50 thermal threshold to uncover drivers of coral thermal tolerance across the Red Sea
S. pistillata displayed a North to South gradient of increasing ED50 thermal thresholds within the Red Sea, though values were lower in Obock than sites in the central Red Sea (Fig. 2, Fig. S6a). Thresholds were lowest in Eilat (34.7 °C ± 0.21; mean ± S.E.M., n = 7), followed by Obock (35.5 °C ± 0.18), compared to the three sites in the central Red Sea (p ≤ 0.03). In turn, thresholds were lower in Al Wajh (36.5 °C ± 0.20) than Al Fahal (37.2 °C ± 0.11; p < 0.05; Table S3). For P. lobata, Fv/Fm thermal thresholds were lowest at the warmest site, Al Qunfudhah (35.7 °C ± 0.11; Fig. 2), compared to all other sites (p < 0.01), except Yanbu (36.5 °C ± 0.25; p = 0.08). Thermal thresholds for A. hemprichii were lowest in Yanbu (36.6 °C ± 0.08) compared to all other sites (p < 0.01; Fig. 2) and lower in Eilat (37.1 °C ± 0.08) compared to Al Fahal (37.7 °C ± 0.10). Lastly, thermal thresholds for P. verrucosa increased with increasing local MMMs across sites (Fig. S6b), with lower thresholds in Eilat (35.2 °C ± 0.20) compared to Al Wajh (36.3 °C ± 0.15) and Obock (36.7 °C ± 0.11; p < 0.03), with no differences among the other sites (p ≥ 0.11; Fig. 2).
Thermal tolerances of four coral species across the Red Sea and Gulf of Aden. Three-parameter log-logistic dose–response curves were fitted to Fv/Fm measurements in response to temperature for each coral species across the study sites, which are plotted with 95% confidence intervals. Vertical lines indicate the mean effective dose 50 (ED50) for each population, with the standard error indicated by the shaded regions based on individual curve fits and ED50s for each colony within each population (each n = 7) to provide an accurate representation of intraspecific biological variability at each site. Letters in the bottom-left corner of the panels represent statistical differences between sites based on Tukey’s post hoc comparisons
Visual bleaching as a low-cost, but less precise, approach to determine thermal thresholds
Calculating ED50 thermal thresholds based on visual assessments of bleaching as a low-cost alternative to measurements of Fv/Fm produced a similar, though less precise outcome. Thermal thresholds determined by visual bleaching for S. pistillata were lower in Eilat (32.4 °C ± 1.97), compared to Obock (36.4 °C ± 0.46) and Al Fahal (39.3 °C ± 0.67; p < 0.05). In turn, thresholds for Al Fahal were significantly higher than Eilat, Al Wajh (35.3 °C ± 0.20), and Yanbu (35.1 °C ± 0.40; p < 0.05; Fig. S3, Table S3). For P. lobata, visual bleaching thermal thresholds were lower in Al Wajh (34.3 °C ± 1.19) than Al Fahal (39.1 °C ± 0.17; p < 0.01) but did not differ significantly between any other sites (p > 0.08; Fig. S3). Log-logistic curves could not be fitted for three colonies from Al Qunfudhah and one colony from Al Fahal, and thus were not included in the analysis. There were no differences between sites for A. hemprichii using visual bleaching thresholds (p > 0.35; Fig. S3), though log-logistic curves could not be fitted for Eilat due to low visual bleaching scores across all temperatures. Lastly, for P. verrucosa, visual bleaching thresholds also were lowest in Eilat (32.9 °C ± 1.34), compared to Al Wajh (36.4 °C ± 0.26), Obock (36.0 °C ± 0.26) and Al Fahal (36.2 °C ± 0.48; p < 0.03). A log-logistic curve could not be fitted for one colony from Al Fahal and was not included in the analysis.
Measurements of algal symbiont density and chlorophyll a metrics in response to acute thermal stress
Traditional metrics of coral bleaching, i.e., symbiont densities and chl a content (McLachlan et al. 2020), were measured at the end of the 18 h assays. For S. pistillata, there was an interactive effect of temperature × site on symbiont densities (p < 0.01; Fig. S4, Table S4), with densities consistent across sites until 39 °C, where densities were 65–93% lower in Eilat than Yanbu and Al Fahal. For chl a (µg cm−2), there also was an interactive effect of temperature × site (p < 0.01; Fig. S5), with chl a lower in Eilat than Yanbu across all temperatures (p < 0.01) and lower than Al Fahal at 33 and 39 °C. In turn, chl a was lower in Al Fahal than Yanbu at 30 °C (p < 0.01).
For P. lobata, symbiont densities and chl a differed between site (p < 0.01) and temperature treatment (p < 0.01), but not the interaction between the two (p ≥ 0.33). Symbiont densities were lower in Yanbu than Al Fahal and Al Qunfudhah (p < 0.05; Fig. S4), while chl a was higher in Al Fahal than Yanbu (p < 0.01).
For A. hemprichii, there was an interactive effect of temperature × site on symbiont densities (p < 0.01), with densities in Eilat consistently lower than Al Wajh and Yanbu across all temperature treatments (Fig. S4). In turn, symbiont densities were lower in Yanbu than Al Wajh in all but the 36 °C treatment (p < 0.01). Chl a was affected by site (p < 0.01) and temperature treatment (p < 0.01), but not the interaction between the two (p = 0.09), with chl a 72% lower in Eilat than Al Wajh and Yanbu (p < 0.01; Fig. S5).
Lastly, there was an interactive effect of temperature × site (p < 0.01) on both symbiont densities and chl a for P. verrucosa (p < 0.01). Symbiont densities were 86% lower in Eilat than Yanbu and Al Wajh at all temperatures but 39 °C (Fig. S4), while chl a was 93% lower in Eilat than Al Wajh across all temperatures (p < 0.01; Fig. S5) and lower than Yanbu at all temperatures but 39 °C (p < 0.01). In turn, chl a was 59% higher in Al Wajh than Yanbu at 36 °C (p < 0.01).
Higher thresholds for corals from the Gulf of Aqaba relative to local thermal environment
Thresholds relative to local conditions (i.e., the MMM at each site) were affected by an interaction of sites × species (p < 0.01; Table S5). Relative thresholds were highest in Eilat for the three coral species sampled, compared to other sites (p < 0.05; Fig. 3a). In the central Red Sea (Al Wajh, Yanbu, and Al Fahal), thresholds relative to local conditions, or ‘relative thresholds’, were consistent across all species (Fig. 3a), with no differences in relative thresholds among these sites for S. pistillata, P. lobata, and A. hemprichii. Relative thresholds for P. verrucosa were lower in Al Fahal compared to Al Wajh and Yanbu (p < 0.05; Fig. 3a). Lastly, relative thresholds for P. lobata sampled in Al Qunfudhah were lower than all other sites (p < 0.01; Fig. 3a).
Thermal thresholds (ED50s) relative to maximum monthly mean (MMM) sea surface temperatures a comparing sites, grouping by species, and b comparing species, grouping by site (mean ± S.E.M.; n = 7). Points represent individual ED50s for each colony. Letters indicate significant differences between: a, sites within species, or b, species within site based on Tukey’s HSD posthoc comparisons, alternating between lower- and upper-case letters for clarity. MMMs for each site are based on NOAA climatology data (Liu et al. 2014)
Comparing relative thresholds within sites indicated lower thresholds for P. verrucosa compared to other species in Yanbu and Al Fahal (p < 0.03; Fig. 3b), though there was no significant difference between P. verrucosa and P. lobata in Yanbu (p = 0.08). Relative thresholds were lower compared to the other species for S. pistillata and P. verrucosa in Eilat and Al Wajh (p < 0.01), though differences with P. lobata in Al Wajh were not significant (p ≥ 0.07). In Obock, relative thresholds were lower for S. pistillata compared to P. verrucosa and P. lobata (p < 0.01). On average, P. verrucosa ranked last of the four study species across sites (thermal tolerance ranking of 1.8 out of 4), ranking last in Al Wajh, Yanbu, and Al Fahal based on the mean Fv/Fm ED50 for each coral population (Fig. 4). Conversely, A. hemprichii was the highest-ranked species on average (thermal tolerance ranking of 3.75 out of 4) and was the highest-ranked species in Eilat, Al Wajh, and Al Fahal.
Heatmap of coral species thermal tolerance ranking at each site, and the average across sites, based on Fv /Fm ED50s (4/purple = highest thermal tolerance, 1/light gray = lowest thermal tolerance). Dark gray squares indicate locations where corals were not sampled. No ranking was produced for Al Qunfudhah as only one species was sampled at that site
Discussion
Evidence of increasing thermal thresholds with increasing maximum monthly mean seawater temperatures
The present study empirically determined the thermal thresholds of four coral species from six sites across the length of the Red Sea using CBASS standardized, acute thermal stress assays. Thermal thresholds, as determined by ED50s, consistently increased with decreasing latitude within the Red Sea (i.e., higher MMMs) across two of the four study species (Stylophora pistillata and Pocillopora verrucosa; Fig. S6) and partially for Porites lobata (excepting some population-specific outliers), providing support for the generalised notion of local adaptation of bleaching limits and thermal thresholds to local seawater temperatures (Jokiel and Brown 2004). Importantly, though a widely accepted notion, this theory has rarely been empirically tested across multiple species and populations within the same study (though see Ulstrup et al. 2006; Jurriaans and Hoogenboom 2019). Here, we conducted standardized multi-species thermal challenges on location at each site to determine ecologically relevant differences in thermal limits across coral populations throughout the Red Sea and Gulf of Aden using acute thermal assays and the Fv/Fm ED50 (Voolstra et al. 2020; Evensen et al. 2021). Still, inconsistent patterns of thermal thresholds for some species, such as P. lobata, highlight the complexities of determining consistent drivers of stress resilience across sites and species.
Based on the Fv/Fm ED50, thermal thresholds were lowest in Eilat, the northernmost and coldest site, for S. pistillata (34.72 °C) and P. verrucosa (35.15 °C), while thresholds were highest at the warmest study site for A. hemprichi, Al Fahal (37.70 °C; Fig. 2). S. pistillata displayed the greatest range (2.5 °C difference) and clearest latitudinal North to South increase in thermal thresholds across the study sites. However, thermal thresholds for S. pistillata in Obock, Djibouti were lower than the three sites in the central/southern Red Sea, despite having a higher MMM (Fig. 1). Larval dispersal models have indicated that coral larvae released in the straits of Bab el Mandeb, near Obock, undergo a northward dispersal through a putative thermal barrier in the southern Red Sea, where MMMs can exceed 32 °C (Fig. 1), thereby selecting for thermally tolerant corals (Fine et al. 2013). Thus, the higher thresholds for S. pistillata populations in the central Red Sea compared to Obock may be a consequence of this thermal selection (Voolstra et al. 2021a; Osman et al. 2018). Additionally, the life history traits of S. pistillata may reflect why this species exhibited clearer latitudinal structure in thermal tolerance than the other species. As a brooder, S. pistillata larvae inherit symbionts from the parents (Rinkevich and Loya 1979) and do not disperse as far as spawners (Ayre and Hughes 2000), which, when combined with shorter generation times than long-lived species such as P. lobata (Harrison and Wallace 1990), could allow for faster acclimation/adaptation of this species to local conditions at each site (Neave et al. 2017). Assessment of additional S. pistillata populations in the southern Red Sea would be interesting to perform (if populations of live coral could be found; see below) to test the potential for thermal selection of recruits from outside the Bab el Mandeb.
Another potential explanation for the lower thermal thresholds for corals from Obock is that these corals were sampled and investigated in February (winter), compared to August (summer) for the other sites. As such, sea surface temperatures (SSTs) were ~ 26.6 °C at the time of the experiments, ~ 4 °C below the local MMM. Conversely, temperatures at the other five sites ranged from 28.2 to 32.1 °C at the time of the experiments, on average 0.82 °C below the local MMMs. Indeed, corals can display bleaching thresholds up to 1 °C lower in the winter compared to summer phenotypes (Berkelmans and Willis 1999), although Fv/Fm ED50 thermal thresholds assessed in a previous study in the winter (February) for S. pistillata corals from Eilat were similar to those reported herein (34.5 °C in the winter versus 34.7 °C in the summer; Evensen et al. 2021); despite seawater temperatures being 22 °C at the time of collection. Thus, the lower thresholds for Obock are likely only partially due to seasonality effects.
Putative long-term effects of bleaching/incomplete recovery in the southern Red Sea
While latitudinal patterns were less obvious for the other three species compared to S. pistillata, thermal thresholds relative to local MMMs generally were consistent across sites, with higher thermal thresholds at sites in the central and southern Red Sea. However, corals from the warmest site in the southern Red Sea, Al Qunfudhah in the Farasan Banks, presented a notable exception. Only one of the four species, P. lobata, was sufficiently abundant to sample in the shallows (< 10 m) at Al Qunfudhah, with the sampled population exhibiting the lowest absolute thermal threshold across all P. lobata populations (35.7 °C), despite originating from the warmest site. In fact, this P. lobata population exhibited the lowest thermal threshold relative to local conditions across all sites and species. Coral populations in this area were strongly impacted by a recent bleaching event in 2015 (Osman et al. 2018), caused by a combination of high temperatures and nutrients (DeCarlo et al. 2020), that resulted in a 44% decrease in coral cover in the region (Monroe et al. 2018; Anton et al. 2020). Indeed, even stress-resistant corals, such as P. lobata, are being pushed beyond their thermal limits, resulting in reduced physiological performance when exposed to repeated heat stress (Klepac and Barshis 2020). Our results support previous indications that corals from sites in the central/southern Red Sea suffered considerable bleaching during marine heatwaves in 2015 (Monroe et al. 2018; Osman et al. 2018; Anton et al. 2020). As such, if the sampled P. lobata colonies from Al Qunfudhah were also affected by these heatwaves, these colonies could still be suffering long-term physiological impacts of the event and/or have been impacted by recurrent thermal stress since. In turn, our inability to collect sufficient replicates of the other species at this site (i.e., a potential lack of remaining species diversity) suggests that corals in this region may be struggling to persist under the rapid warming of the Red Sea (Cantin et al. 2010; Raitsos et al. 2011).
High relative, but not absolute, thresholds in Gulf of Aqaba corals
In the northern Red Sea, corals in Eilat displayed exceptionally high tolerances relative to their environment. Despite 2.7–4.6 °C lower MMM temperatures in Eilat compared to the other study sites, thermal thresholds for corals in Eilat were only 0–1.4 °C lower than the other sites, resembling results found previously using CBASS (Voolstra et al. 2021a). As a result, thresholds relative to the MMM were highest for the three coral species studied in Eilat (Fig. 3a). These results support previous findings that corals from the northern Red Sea are capable of tolerating temperatures well above those experienced in the GoA, possibly through physiological trade-offs in which energy reserves and biomass are maintained during thermal stress in exchange for reduced calcification rates (Grottoli et al. 2017). The higher thermal tolerance is presumed to be due to prior migration through the much warmer waters in the southern Red Sea before reaching the region (Fine et al. 2013; Osman et al. 2018), with higher thermal tolerances for corals in the region retained via historical selection and/or continued larval supply from more thermally tolerant individuals further South. In turn, the presence of genetically distinct Symbiodiniaceae and differences in microbial assembly likely also contribute to overall stress resilience for corals in the region (Neave et al. 2017; Hume et al. 2020; Osman et al. 2020; Voolstra et al. 2020, 2021a; Savary et al. 2021).
An important nuance to previous studies exposing this phenomenon, however, is that the absolute thresholds herein were lower in Eilat than the other sites (also see Voolstra et al. 2021a), indicating that these corals could be losing thermal tolerance over evolutionary time by acclimating to the cooler climate in the GoA, or simply represent a distinct population altogether. The latter is supported by a recent study that used a transcriptome-wide single nucleotide polymorphism (SNP) analysis to show that GoA and central Red Sea Stylophora pistillata represent clearly distinct lineages, though the pattern was not resolved unambiguously (Voolstra et al. 2021a) and GoA S. pistillata are from the same genetic sub-clade as central Red Sea populations (Malik et al. 2021). The lower absolute thresholds for GoA corals provide further rationale for a standardized approach to determining thermal thresholds in corals, ideally in combination with accompanying population genetic data, as most comparisons of tolerance between regions to date are relative to local conditions (i.e., based on MMMs). This can be misleading for conservation and restoration efforts across large spatial scales and the identification of true ‘super’ corals and coral refugia (Voolstra et al. 2021b; Cacciapaglia and Woesik 2015; Camp et al. 2018).
Species-specific responses of corals to acute thermal stress
Beyond regional comparisons, our approach allowed for direct comparisons of thermal sensitivities across species within the studied reefs to compare to in situ bleaching observations of these four coral genera. To date, the common approach of determining bleaching thresholds using degree-heating weeks (DHWs) yields a single threshold (1 °C above the local MMM) for all species across large areas of reef (5 km resolution; Liu et al. 2014), yet there is extensive evidence that coral species within sites exhibit different responses and tolerances to thermal stress (Loya et al. 2001; van Woesik et al. 2011; Pineda et al. 2013; Hughes et al. 2017; Voolstra et al. 2020). Consistent with previous in situ bleaching observations highlighting the sensitivity of the genus Pocillopora to thermal stress (Marshall and Baird 2000; Loya et al. 2001), P. verrucosa had the lowest thresholds of the four species, ranking last at the three central Red Sea sites where all coral species were sampled (Fig. 4). Conversely, and surprisingly, A. hemprichii displayed the highest overall thresholds among the four species sampled and was only outperformed at Yanbu by S. pistillata. Reports of the sensitivity of Acropora to thermal stress are mixed (Marshall and Baird 2000; Loya et al. 2001; Hughes et al. 2017; Dalton et al. 2020), which may be a reflection of the high diversity of species and morphologies within this genus (Baird and Marshall 2002; Hughes et al. 2017) or be due to contrasting responses of this genus to thermal stress events of different intensities and durations. Still, while Acropora may not always be susceptible to bleaching, this genus typically experiences considerable mortality and struggles to re-establish itself following bleaching events (Baird and Marshall 2002; Burt et al. 2011). Thus, it becomes important to consider differences between thermal stress response and post-stress resilience in future experimental designs (Savary et al. 2021).
A closer look at the shape of the Fv/Fm ED50 dose–response curves for A. hemprichii may offer insight into the disassociation between prior reports of bleaching susceptibility and mortality. A. hemprichii corals experienced little decrease in Fv/Fm up to 36 °C, before decreasing sharply at 39 °C, indicating high initial tolerance to acute thermal stress, but a high susceptibility past a certain threshold. In turn, while P. lobata may have been marginally outperformed by A. hemprichii at the sites where both species were present, it was the only species remaining in abundance at the warmest assayed site in the southern Red Sea. Similarly, in the nearby Persian/Arabian Gulf, extreme bleaching at some sites has resulted in low species diversity, with the genus Porites dominating (88% of coral cover) the post-bleaching community (Burt et al. 2011). Thus, while P. lobata may be sensitive to bleaching and acute thermal stress, this species does not typically suffer from high rates of mortality (Marshall and Baird 2000; Baird and Marshall 2002) and can display remarkable persistence and recovery from thermal stress events (van Woesik et al. 2011). Indeed, response patterns of corals to acute thermal assays may not necessarily accurately reflect patterns of recovery and long-term survival, with differences in tissue thickness, energy reserves, and associated symbiont and microbial communities among a myriad of traits that can influence both the stress response and recovery potential of corals (Ziegler et al. 2017, 2019). As such, understanding how species-specific thermal tolerances to acute thermal stress and bleaching susceptibility interact with survival and long-term population dynamics is crucial to predicting the response of coral communities to global warming.
The F v /F m ED50 and acute stress assays as a standardized approach to diagnose coral bleaching susceptibility
Our standardized approach, combining acute thermal assays and non-destructive measurements of photosynthetic efficiency (Fv/Fm) in a portable experimental system, the CBASS, resolved multiple putative drivers of variation in coral thermal tolerances, including historical/latitudinal climate, possible remnant impacts of recent thermal stress events, and species-specific response patterns. The study focused on Fv/Fm as a key response variable, with previous evidence that this metric can serve as a proxy for holobiont performance in response to both acute and chronic thermal exposures (Voolstra et al. 2020; Evensen et al. 2021) and resolve historical differences in bleaching susceptibility between conspecific corals from inshore and offshore sides of the same reef (Voolstra et al. 2020). Declines in Fv/Fm are expected to occur rapidly at the onset of heating as photosystem light-harvesting complexes are highly sensitive to thermal damage, with tolerance of the symbionts to heat stress putatively representative of coral bleaching susceptibility (Warner et al. 1999). Conversely, common bleaching metrics, such as symbiont densities and chl a, are integrative responses that follow a suite of cellular pathways (Weis 2008), and thus, reaction to acute thermal stress may not be as clear or immediate and may be less suited for acute thermal assays. This is further supported by recent work demonstrating that the effects of heat stress manifest long before differences in symbiont densities or chl a are apparent (Rädecker et al. 2021). Site-specific differences in thermal tolerance were resolved using visual bleaching assessments for S. pistillata, though at a coarser resolution than Fv/Fm (Fig. S3), corroborating the usefulness of Fv/Fm measurements as a representative proxy of bleaching susceptibility (Voolstra et al. 2020). Nonetheless, further research is necessary to resolve how differences between measurements of Fv/Fm, chl a, symbiont densities, and visual bleaching relate to differences in coral thermal thresholds in situ and improve the use of acute thermal assays as a diagnostic tool to assess the natural bleaching susceptibility of corals (Voolstra et al. 2021b).
In addition to resolving how different physiological variables respond to acute thermal stress, it is crucial to determine how the observed responses to acute thermal stress relate to population- and species-specific bleaching thresholds and susceptibilities in response to natural thermal stress events as well as long-term recovery and survival. Indeed, the thermal thresholds determined herein should not be considered as absolute measures or values, but rather as standardized and rapid diagnostic proxies for ecological and evolutionary variation in thermotolerance of reef-building corals that may also be indicative of longer-term thermal stress resilience (Voolstra et al. 2020; Evensen et al. 2021). Moreover, the thresholds should be considered context-dependent values for each colony or population, as differing light intensities, nutrient concentrations, and many other factors can influence the physiological responses and bleaching susceptibility of corals to thermal stress (Warner et al. 1999; DeCarlo et al. 2020; Suggett and Smith 2020). Lastly, an additional consideration for future studies using this approach will be the appropriate selection of baseline and experimental temperature treatments. Although similar Fv/Fm ED50s were produced for S. pistillata in Eilat herein using 30 °C as a baseline temperature compared to a previous study using the local MMM (27 °C) as a baseline temperature (34.7 vs. 34.5 °C, respectively; Evensen et al. 2021), using site-specific baseline temperatures that match site MMMs (e.g., 27 °C for Eilat vs. 30 °C for Al Wajh) is likely best practice for future studies where the range of MMMs may exceed that of sites from the present study.
Overall, much like a cardiac stress test to diagnose potential for coronary artery disease in humans (Gianrossi et al. 1989), our results demonstrate that standardized short-term thermal stress assays can be used to determine and compare relative thermotolerances of corals across broad taxonomic and spatial scales. Notably, this approach could identify heat tolerant individuals and populations for subsequent elucidation of the environmental drivers of and molecular mechanisms underlying increased stress resilience, as well as prioritising specific coral populations for conservation and restoration efforts (Voolstra et al. 2021b).
Data availability
All code and data used in the statistical analyses are available in the Supplementary Material and the electronic notebook associated with this publication on the Barshis Lab GitHub: https://github.com/BarshisLab/Gradient-physiology.
References
Anton A, Randle JL, Garcia FC, Rossbach S, Ellis JI, Weinzierl M, Duarte CM (2020) Differential thermal tolerance between algae and corals may trigger the proliferation of algae in coral reefs. Glob Change Biol 26:4316–4327
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci 110:1387–1392
Berkelmans R, Willis BL (1999) Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18:219–228
Berumen ML, Voolstra CR, Daffonchio D, Agusti S, Aranda M, Irigoien X et al (2019) The red sea: environmental gradients shape a natural laboratory in a Nascent Ocean. In: Voolstra CR, Berumen ML (eds) Coral reefs of the red sea. Springer International Publishing, Cham, pp 1–10. https://doi.org/10.1007/978-3-030-05802-9_1
Burt J, Al-Harthi S, Al-Cibahy A (2011) Long-term impacts of coral bleaching events on the world’s warmest reefs. Mar Environ Res 72:225–229
Cacciapaglia C, van Woesik R (2015) Reef-coral refugia in a rapidly changing ocean. Glob Change Biol 21:2272–2282
Camp EF, Schoepf V, Suggett DJ (2018) How can “Super Corals” facilitate global coral reef survival under rapid environmental and climatic change? Glob Change Biol 24:2755–2757
Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC (2010) Ocean warming slows coral growth in the central Red Sea. Science 329:322–325
Dalton SJ, Carroll AG, Sampayo E, Roff G, Harrison PL, Entwistle K, Huang Z, Salih A, Diamond SL (2020) Successive marine heatwaves cause disproportionate coral bleaching during a fast phase transition from El Niño to La Niña. Sci Total Environ 715:136951
DeCarlo TM, Gajdzik L, Ellis J, Coker DJ, Roberts MB, Hammerman NM, Pandolfi JM, Monroe AA, Berumen ML (2020) Nutrient-supplying ocean currents modulate coral bleaching susceptibility. Sci Adv 6:eabc5493. https://doi.org/10.1126/sciadv.abc5493
Dixon GB, Davies SW, Aglyamova GV, Meyer E, Bay LK, Matz MV (2015) Genomic determinants of coral heat tolerance across latitudes. Science 348:1460–1462
Evensen NR, Fine M, Perna G, Voolstra CR, Barshis DJ (2021) Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol Oceanogr 66:1718–1729
Fine M, Gildor H, Genin A (2013) A coral reef refuge in the Red Sea. Glob Change Biol 19:3640–3647
Gianrossi R, Detrano R, Mulvihill D, Amar K-O, Dubach P, Colombo A, McArthur D, Froelicher V (1989) Exercise-induced ST depression in the diagnosis of coronary artery disease. Meta-Anal Circ 80:87–98
Grottoli AG, Tchernov D, Winters G (2017) Physiological and biogeochemical responses of super-corals to thermal stress from the Northern Gulf of Aqaba, Red Sea. Front Mar Sci 4:215. https://doi.org/10.3389/fmars.2017.00215
Grottoli AG, Toonen RJ, Woesik R van, Thurber RV, Warner ME, McLachlan RH, Price JT, Bahr KD, Baums IB, Castillo K, Coffroth MA, Cunning R, Dobson K, Donahue M, Hench JL, Iglesias‐Prieto R, Kemp DW, Kenkel CD, Kline DI, Kuffner IB, Matthews JL, Mayfield A, Padilla‐Gamino J, Palumbi S, Voolstra CR, Weis VM, Wu HC (2021) Increasing comparability among coral bleaching experiments. Ecol Appl 31:e02262. https://doi.org/10.1002/eap.2262
Harrison P, Wallace C (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Ecosystems of the World 25: Coral Reefs. Elsevier Science, Amsterdam, Netherlands, pp 133–207
Holmes G, Ortiz J, Kaniewska P, Johnstone R (2008) Using three-dimensional surface area to compare the growth of two Pocilloporid coral species. Mar Biol 155:421–427
Howells EJ, Abrego D, Meyer E, Kirk NL, Burt JA (2016) Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob Change Biol 22:2702–2714
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018a) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018b) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Kerry JT, Connolly SR, Baird AH, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Jacobson M, Liu G, Pratchett MS, Skirving W, Torda G (2019) Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat Clim Change 9:40
Hume BCC, Mejia-Restrepo A, Voolstra CR, Berumen ML (2020) Fine-scale delineation of Symbiodiniaceae genotypes on a previously bleached central Red Sea reef system demonstrates a prevalence of coral host-specific associations. Coral Reefs 39:583–601
Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob Change Biol 10:1627–1641
Jurriaans S, Hoogenboom MO (2019) Thermal performance of scleractinian corals along a latitudinal gradient on the Great Barrier Reef. Philos Trans R Soc B Biol Sci 374:20180546
Klepac CN, Barshis DJ (2020) Reduced thermal tolerance of massive coral species in a highly variable environment. Proc R Soc B Biol Sci 287:20201379
Kleypas J, Allemand D, Anthony K, Baker AC, Beck MW, Hale LZ, Hilmi N, Hoegh-Guldberg O, Hughes T, Kaufman L, Kayanne H, Magnan AK, Mcleod E, Mumby P, Palumbi S, Richmond RH, Rinkevich B, Steneck RS, Voolstra CR, Wachenfeld D, Gattuso JP (2021) Designing a blueprint for coral reef survival. Biol Conserv 257:109107. https://doi.org/10.1016/j.biocon.2021.109107
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biol 28(16):2570–2580.e6. https://doi.org/10.1016/j.cub.2018.07.008
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2020) emmeans: estimated marginal means, aka least-squares means
Liu G, Heron SF, Eakin CM, Muller-Karger FE, Vega-Rodriguez M, Guild LS, De La Cour JL, Geiger EF, Skirving WJ, Burgess TFR, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sens 6:11579–11606
Lough JM, Anderson KD, Hughes TP (2018) Increasing thermal stress for tropical coral reefs: 1871–2017. Sci Rep 8:6079
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Malik A, Einbinder S, Martinez S, Tchernov D, Haviv S, Almuly R, Zaslansky P, Polishchuk I, Pokroy B, Stolarski J, Mass T (2021) Molecular and skeletal fingerprints of scleractinian coral biomineralization: from the sea surface to mesophotic depths. Acta Biomater 120:263–276
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
McLachlan RH, Price JT, Solomon SL, Grottoli AG (2020) Thirty years of coral heat-stress experiments: a review of methods. Coral Reefs 39:885–902
Monroe AA, Ziegler M, Roik A, Röthig T, Hardenstine RS, Emms MA, Jensen T, Voolstra CR, Berumen ML (2018) In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015/2016 global coral bleaching event. PLoS ONE 13:e0195814
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc Natl Acad Sci 116:10586–10591
Neave MJ, Rachmawati R, Xun L, Michell CT, Bourne DG, Apprill A, Voolstra CR (2017) Differential specificity between closely related corals and abundant Endozoicomonas endosymbionts across global scales. ISME J 11:186–200
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Osman EO, Smith DJ, Ziegler M, Kürten B, Conrad C, El-Haddad KM, Voolstra CR, Suggett DJ (2018) Thermal refugia against coral bleaching throughout the northern Red Sea. Glob Change Biol 24:e474–e484
Osman EO, Suggett DJ, Voolstra CR, Pettay DT, Clark DR, Pogoreutz C, Sampayo EM, Warner ME, Smith DJ (2020) Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities. Microbiome 8:8
Pineda J, Starczak V, Tarrant A, Blythe J, Davis K, Farrar T, Berumen M, da Silva JCB (2013) Two spatial scales in a bleaching event: corals from the mildest and the most extreme thermal environments escape mortality. Limnol Oceanogr 58:1531–1545
R Core Team (2018) R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/
Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina J-B, Meibom A, Voolstra CR (2021) Heat stress destabilizes symbiotic nutrient cycling in corals. Proc Natl Acad Sci 118:e2022653118. https://doi.org/10.1073/pnas.2022653118
Raitsos DE, Hoteit I, Prihartato PK, Chronis T, Triantafyllou G, Abualnaja Y (2011) Abrupt warming of the Red Sea. Geophys Res Lett 38:L14601
Rinkevich B, Loya Y (1979) The reproduction of the Red Sea coral Stylophora pistillata. I. Gonads and planulae. Mar Ecol Prog Ser 1:133–144
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:e0146021
Savary R, Barshis DJ, Voolstra CR, Cárdenas A, Evensen NR, Banc-Prandi G, Fine M, Meibom A (2021) Fast and pervasive transcriptomic resilience and acclimation of extremely heat-tolerant coral holobionts from the northern Red Sea. Proc Natl Acad Sci 118:e2023298118. https://doi.org/10.1073/pnas.2023298118
Schoepf V, Stat M, Falter JL, McCulloch MT (2015) Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci Rep 5:17639
Skirving WJ, Heron SF, Marsh BL, Liu G, De La Cour JL, Geiger EF, Eakin CM (2019) The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs 38:547–557
Stimson J, Kinzie RA (1991) The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol 153:63–74
Suggett DJ, Smith DJ (2020) Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob Change Biol 26:68–79
Ulstrup KE, Berkelmans R, Ralph PJ, van Oppen MJH (2006) Variation in bleaching sensitivity of two coral species across a latitudinal gradient on the Great Barrier Reef: the role of zooxanthellae. Mar Ecol Prog Ser 314:135–148
van Hooidonk R, Maynard J, Tamelander J, Gove J, Ahmadia G, Raymundo L, Williams G, Heron SF, Planes S (2016) Local-scale projections of coral reef futures and implications of the Paris agreement. Sci Rep 6:1–8
van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76
Vinagre C, Leal I, Mendonça V, Madeira D, Narciso L, Diniz MS, Flores AAV (2016) Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol Indic 62:317–327
Voolstra CR, Buitrago-Lopez C, Perna G, Cardenas A, Hume B, Radecker N, Barshis DJ (2020) Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob Change Biol 26:4328–4343
Voolstra CR, Valenzuela JJ, Turkarslan S, Cárdenas A, Hume BCC, Perna G, Buitrago-López C, Rowe K, Orellana MV, Baliga NS, Paranjape S, Banc-Prandi G, Bellworthy J, Fine M, Frias-Torres S, Barshis DJ (2021a) Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol Ecol 30:4466–4480
Voolstra CR, Suggett DJ, Peixoto RS, Parkinson JE, Quigley KM, Silveira CB, Sweet M, Muller EM, Barshis DJ, Bourne DG, Aranda M (2021b) Extending the natural adaptive capacity of coral holobionts. Nature Rev Earth Environ 2(11):747–762 https://doi.org/10.1038/s43017-021-00214-3
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci 96:8007–8012
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211:3059–3066
Wickham H (2016) ggplot2: elegant Graphics for Data Analysis. Springer, New York
Winters G, Holzman R, Blekhman A, Beer S, Loya Y (2009) Photographic assessment of coral chlorophyll contents: Implications for ecophysiological studies and coral monitoring. J Exp Mar Biol Ecol 380:25–35
Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR (2017) Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Commun. 8:14213. https://doi.org/10.1038/ncomms14213
Ziegler M, Grupstra CGB, Barreto MM, Eaton M, BaOmar J, Zubier K, Al-Sofyani A, Turki AJ, Ormond R, Voolstra CR (2019) Coral bacterial community structure responds to environmental change in a host-specific manner. Nature Commun. 10:3092. https://doi.org/10.1038/s41467-019-10969-5
Acknowledgments
We thank Jessica Bellworthy, Dror Komet, and all the skippers and boat crews for their invaluable help and support, as well as the reviewers for providing valuable feedback that improved the manuscript. We also thank the Direction de l'Environnement et de Développement Durable (DEDD) and Ministry of the Environment and Sustainable Development (MEDD) for their critical assistance in supporting the research in Djibouti, particularly Mme Bilan Hassan Ismail and Dr. Youssouf Moussa Omar, through coordination of the research, permits, and sample transport, as well as Chris Canellakis and Omar Awaleh from the US Embassy for their logistical support. This research was supported by funding from the U.S. Israeli Binational Science foundation (award 2016403 to DJB and MF), National Science Foundation (award 1833201 to DJB), and by the University of Konstanz and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 433042944 (to CRV). On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CRV and DJB conceived and designed the study. All co-authors performed the experiments and collected the data. NRE analyzed the data and wrote the manuscript, with substantial contributions to drafting the manuscript by CRV and DJB. All co-authors approved the final version of the paper.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evensen, N.R., Voolstra, C.R., Fine, M. et al. Empirically derived thermal thresholds of four coral species along the Red Sea using a portable and standardized experimental approach. Coral Reefs 41, 239–252 (2022). https://doi.org/10.1007/s00338-022-02233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02233-y