Abstract
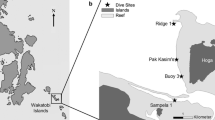
Marine community datasets are key to the effective management and conservation of marine ecosystems, including coral reefs, which are increasingly threatened by a myriad of stressors. Although community information exists for many comparatively well-studied taxa, other common groups remain to be examined for even such basic information. In this study, we report on the zoantharian communities (Cnidaria: Anthozoa: Hexacorallia: Zoantharia) on the reefs of Bonaire in the southern Caribbean, and compare current results from 30, 20, 10, and 5 m depths to recent similar surveys from nearby Curaçao. The surveys revealed a total of 17 zoantharian species and epibiotic associations on the reefs of Bonaire. Additionally, results showed that while zoantharian assemblages around Bonaire at shallow 5 and 10 m depths, dominated by Palythoa spp., were similar to those found on Curaçao, diversity and numbers of zoantharians were higher at 20 and 30 m due to more abundant epibiotic Parazoanthidae species associated with sponges. Differences in assemblage structure were seen in deeper 20 and 30 m depths between the two islands, implying that conservation of deeper reef slopes, or along depth gradients, may need to be independently considered and addressed for each location. Analyses with environmental parameters on the Bonaire dataset indicate the potential importance of coral reef rugosity and physical structure in shaping these zoantharian communities, aspects that should be focused on in more detail in future research.






Similar content being viewed by others
References
Alcolado PM (2004) General comments on species inventory, fisheries, culture and some community features of the Porifera in Cuba. BMIB – Bolletino dei Musei e degli Istituti Biologici 68:175–186
Alvarez B, van Soest RW, Rützler K (1998) A revision of the Axinellidae (Porifera: Demospongiae) of the Central-West Atlantic region. Smithson Contrib Zool 598:1–47
Amato DW, Bishop JM, Glenn CR, Dulai H, Smith CM (2016) Impact of submarine groundwater discharge on marine water quality and reef biota of Maui. PLoS One 11:e0165825
Bak RPM, Nieuwland G (1995) Long-term change in coral communities along depth gradients over leeward reefs in the Netherlands Antilles. Bull Mar Sci 56:609–619
Bak RPM, Nieuwland G, Meesters EH (2005) Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curaçao and Bonaire. Coral Reefs 24:475–479
Bastidas C, Bone D (1996) Competitive strategies between Palythoa caribaeorum and Zoanthus sociatus (Cnidaria: Anthozoa) at a reef flat environment in Venezuela. Bull Mar Sci 59:543–555
Belford SG, Phillip DA (2012) Intertidal distribution patterns of zoanthids compared to their scleractinian counterparts in the southern Caribbean. Int J Oceanog Mar Ecol Sys 1:67–75
Burke L, Maidens J (2004) Reefs at risk in the Caribbean. World Resources Institute, Washington, DC, pp 1–81
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. J Exp Mar Biol Ecol 366:56–69
Condor-Lujan B, Louzada T, Hajdu E, Klautau M (2018) Morphological and molecular taxonomy of calcareous sponges (Porifera: Calcarea) from Curaçao, Caribbean Sea. Zool J Linn Soc 183:459–525
Cramer KL, Jackson JB, Donovan MK, Greenstein BJ, Korpanty CA, Cook GM, Pandolfi JM (2020) Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci Adv 6:eaax9395
Crocker LA, Reiswig HM (1981) Host specificity in sponge-encrusting Zoanthidea (Anthozoa: Zoantharia) of Barbados, West Indies. Mar Biol 65:231–236
Cruz IC, Waters LG, Kikuchi RK, Leão ZM, Turra A (2018) Marginal coral reefs show high susceptibility to phase shift. Mar Poll Bull 135:551–561
De Bakker DM, Meesters EH, Bak RPM, Nieuwland G, Van Duyl FC (2016) Long-term shifts in coral communities on shallow to deep reef slopes of Curaçao and Bonaire: Are there any winners? Front Mar Sci 3:247
De Bakker DM, Van Duyl FC, Bak RPM, Nugues MN, Nieuwland G, Meesters EH (2017) 40 years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 36:355–367
De Bakker DM, Van Duyl FC, Perry CT, Meesters EH (2019) Extreme spatial heterogeneity in carbonate accretion potential on a Caribbean fringing reef linked to local human disturbance gradients. Global Change Biol 25:4092–4104
de Voogd NJ, Alvarez B, Boury-Esnault N, Carballo JL, Cárdenas P, Díaz MC, Dohrmann M, Downey R, Hajdu E, Hooper JNA, Kelly M, Klautau M, Manconi R, Morrow CC, Pisera AB, Ríos P, Rützler K, Schönberg C, Vacelet J, van Soest RWM (2021) World Porifera Database. Niphates Duchassaing & Michelotti, 1864. Accessed at: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=166770 on 2021–11–14
Duchassaing de Fonbressin P, Michelotti J (1860) Mémoire sur les Coralliaires des Antilles. Extrait des Mémoires de l’Académie des Sciences de Turin. Série II. Tome XIX. L’Imprimerie Royale, Turin, 89 pp, pls 1–10
Duchassaing de Fonbressin P, Michelotti J (1864) Spongiaires de la mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem. 21:1−124, pls I−XXV
Duerden JE (1898) Jamaican Actiniaria. Part I.—Zoantheae. Sci Trans R Dublin Soc (Ser 2) 6:329–384
Duerden JE (1900) Jamaican Actiniaria. Part II. Stichodactylinae and Zoantheae. Sci Trans Roy Dublin Soc (Ser 2) 7:133–200
Duran A, Shantz AA, Burkepile DE, Collado-Vides L, Ferrer VM, Palma L, Ramos A, González-Díaz P (2018) Fishing, pollution, climate change, and the long-term decline of coral reefs off Havana. Cuba Bull Mar Sci 94:213–228
Van Duyl FC (1985) Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Natuurwetenschappelijke Studiekring voor Suriname en de Nederlandse Antillen, Utrecht, pp. i–v, 1–37, maps C1–44, B1–37, legend
Edgar GJ, Bates AE, Bird TJ, Jones AH, Kininmonth S, Stuart-Smith RD, Webb TJ (2016) New approaches to marine conservation through the scaling up of ecological data. Ann Rev Mar Science 8:435–461
Ellis J (1768) An account of the Actinia sociata, or clustered animal-flower, lately found on the sea-coasts of the new-ceded islands: In a letter from John Ellis, Esquire, F.R.S. to the Right Honourable the Earl of Hillsborough F.R.S. Phil Trans 57:428–437
Gladfelter WB, Ogden JC, Gladfelter EH (1980) Similarity and diversity among coral reef fish communities: a comparison between tropical western Atlantic (Virgin Islands) and tropical central Pacific (Marshall Islands) patch reefs. Ecology 61:1156–1168
Goreau TF (1959) The ecology of Jamaican coral reefs I. Species Composition and Zonation Ecology 40:67–90
Higgin T (1877) XXIV.—Description of some sponges obtained during a cruise of the steam-yacht ‘Argo’in the Caribbean and neighbouring seas. J Nat Hist 19:291–299
Hill CEL, Lymperaki MM, Hoeksema BW (2021) A centuries-old manmade reef in the Caribbean does not substitute natural reefs in terms of species assemblages and interspecific competition. Mar Pollut Bull 169:112576
Hill J, Wilkinson CL (2004) Methods for ecological monitoring of coral reefs. Australian Institute of Marine Science, Townsville.
Hoeksema BW, Koh EG (2009) Depauperation of the mushroom coral fauna (Fungiidae) of Singapore (1860s–2006) in changing reef conditions. Raffles Bull Zool Suppl 22:91–101
Hoeksema BW, García-Hernández JE, van Moorsel GWNM, Olthof G, ten Hove HA (2020) Extension of the recorded host range of Caribbean Christmas tree worms (Spirobranchus spp.) with two scleractinians, a zoantharian, and an ascidian. Diversity 12:115
Holt BG, Rioja-Nieto R, MacNeil MA, Lupton J, Rahbek C (2013) Comparing diversity data collected using a protocol designed for volunteers with results from a professional alternative. Methods Ecol Evol 4:383–392
Kemp DW, Cook CB, LaJeunesse TC, Brooks WR (2006) A comparison of the thermal bleaching responses of the zoanthid Palythoa caribaeorum from three geographically different regions in south Florida. J Exp Mar Biol Ecol 335:266–276
Ladd MC, Shantz AA, Burkepile DE (2019) Newly dominant benthic invertebrates reshape competitive networks on contemporary Caribbean reefs. Coral Reefs 38:1317–1328
LaJeunesse TJ (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
Lamarck JB de (1814 [1813]) Sur les polypiers empâtés. Ann Mus Hist Nat Paris 20:294–312;370–386;432–458
Linnaeus C (1759) Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus II. Editio decima, reformata, pp. [1-4], 825-1384. Holmiæ. (L. Salvii).
Le Sueur CA (1817) Observations on several species of the genus Actinia: Illustrated by figures. J Acad Nat Sci Phil 1:149–189
Lesser MP, Slattery M (2018) Sponge density increases with depth throughout the Caribbean. Ecosphere 9:e02525
Lewis SM (1982) Sponge-zoanthid associations: functional interactions. Smithson Contrib Mar Sci 12:465–474
Lincoln Smith MP (1988) Effects of observer swimming speed on sample counts of temperate rocky reef fish assemblages. Mar Ecol Prog Ser 43:223–231
Martinez Arbizu P (2020) pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.4
McWilliams JP, Côté IM, Gill JA, Sutherland WJ, Watkinson AR (2005) Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology 8:2055–2060
Montano S, Reimer JD, Ivanenko VN, García-Hernández JE, van Moorsel GWNM, Galli P, Hoeksema BW (2020) Widespread occurrence of a rarely known association between the hydrocorals Stylaster roseus and Millepora alcicornis at Bonaire, southern Caribbean. Diversity 12:218
Montenegro J, Hoeksema BW, Santos ME, Kise H, Reimer JD (2020) Zoantharia (Cnidaria: Hexacorallia) of the Dutch Caribbean and one new species of Parazoanthus. Diversity 12:190
Montenegro-Gonzalez JA, Acosta A (2010) Habitat preference of Zoantharia genera depends on host sponge morphology. Univ Sci 15:110–121
Munro C (2005) Diving systems. In: Eleftheriou A, McIntyre A (eds) Methods for the study of marine benthos, 3rd edn. Blackwell Science, Oxford, pp 112–159
Newman MJ, Paredes GA, Sala E, Jackson JB (2006) Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett 9:1216–1227
Oksanen J (2015) Multivariate analysis of ecological communities in R. Retrieved from http://cc.oulu.fi/~jarioksa/opetus/metodi/vegantutor.pdf (accessed 30 August 2018)
Pang RK (1973) The systematics of some Jamaican excavating sponges (Porifera). Postilla 161:1–75
Pinheiro HT, Goodbody-Gringley G, Jessup ME, Shepherd B, Chequer AD, Rocha LA (2016) Upper and lower mesophotic coral reef fish communities evaluated by underwater visual censuses in two Caribbean locations. Coral Reefs 35:139–151
Pomponi SA, Díaz MC, Van Soest RWM, Bell LJ, Busutil L, Gochfeld DJ, Kelly M. Slattery M (2019) Sponges. In: Loya Y, Puglise KA, Bridge T (eds) Mesophotic coral ecosystems of the world. Springer, New York, pp 563–588
Pulitzer-Finali G (1986) A collection of West Indian Demospongiae (Porifera): In Appendix, a list of the Demospongiae hitherto recorded from the West Indies. Montotipia Erredi, 1986.
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Reimer JD, Fujii T (2017) Zoantharia (Cnidaria: Anthozoa: Hexacorallia) diversity research in Japan: current state and future trends. In: Motokawa M, Kajihara H (eds) Species diversity of animals in Japan. Springer, Tokyo, pp 383–399
Reimer JD, Foord C, Irei Y (2012) Species diversity of shallow water zoanthids (Cnidaria: Anthozoa: Hexacorallia) in Florida. J Mar Biol 2012:856079
Reimer JD, Poliseno A, Hoeksema BW (2014) Shallow-water zoantharians (Cnidaria, Hexacorallia) from the Central Indo-Pacific. ZooKeys 444:1–57
Reimer JD, Wee HB, García-Hernández JE, Hoeksema BW (2018) Zoantharia (Anthozoa: Hexacorallia) abundance and associations with Porifera and Hydrozoa across a depth gradient on the west coast of Curaçao. System Biodivers 16:820–830
Roberts CM, McClean CJ, Veron JE, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284
RStudio Team (2020) RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/
Santos ME, Faria-Junior E, Aued AW, Peluso L, Kitahara MV, Pires DO, Zilberberg C (2020) Benthic Cnidaria community in the oceanic archipelago of Trindade and Martin Vaz, southwestern Atlantic Ocean. Reg Stud Mar Sci 33:100895
Schmidt O (1870) Grundzüge einer Spongien-Fauna des atlantischen Gebietes. Wilhelm Engelmann, Leipzig, iii-iv, 1–88, pls I-VI
Schmitt E, Sluka R, Sullivan-Sealey K (2002) Evaluating the use of roving diver and transect surveys to assess the coral reef fish assemblage off southeastern Hispaniola. Coral Reefs 21:216–223
Sebens KP (1982) Intertidal distribution of zoanthids on the Caribbean coast of Panama: effects of predation and desiccation. Bull Mar Sci 32:316–335
Shantz AA, Ladd MC, Burkepile DE (2020) Overfishing and the ecological impacts of extirpating large parrotfish from Caribbean coral reefs. Ecol Monogr 90:e01403
Sheppard C, Rioja-Nieto R (2005) Sea surface temperature 1871–2099 in 38 cells in the Caribbean region. Mar Environ Res 60:389–396
Suchanek TH, Green DJ (1981) Interspecific competition between Palythoa caribaeorum and other sessile invertebrates on St. Croix Reefs, U.S Virgin Islands. Proc 4th Int Coral Reef Symp 2:679–684
Swain TD (2009) Isozoanthus antumbrosus, a new species of zoanthid (Cnidaria: Anthozoa: Zoanthidea) symbiotic with Hydrozoa from the Caribbean, with a key to hydroid and sponge-symbiotic zoanthid species. Zootaxa 2051:41–48
Swain TD (2012) Context-dependent effects of symbiosis: Zoanthidea colonization generally improves Demospongiae condition in native habitats. Mar Biol 159:1429–1438
Swain T, Wulff J (2006) Diversity and specificity of Caribbean sponge-zoanthid symbioses: a foundation for understanding the adaptive significance of symbioses and generating hypotheses about higher-order systematics. Biol J Linn Soc 92:695–711
Swain TD, Wulff JL (2007) Diversity and specificity of Caribbean sponge–zoanthid symbioses: a foundation for understanding the adaptive significance of symbioses and generating hypotheses about higher-order systematics. Biol J Linn Soc 92:695–711
Tsounis G, Edmunds PJ, Bramanti L, Gambrel B, Lasker HR (2018) Variability of size structure and species composition in Caribbean octocoral communities under contrasting environmental conditions. Mar Biol 165:29
Van Soest RW (1980) Marine sponges from Curaçao and other Caribbean localities Part II. Haplosclerida Stud Fauna Curaçao Caribbean is 62:1–73
Varela C, Guitart B, Ortiz M, Lalana R (2002) Los zoantideos (Cnidaria, Anthozoa, Zoanthiniaria), de la region occidental de Cuba. Rev Invest Mar 23:179–184
Velásquez J, Sánchez JA (2015) Octocoral species assembly and coexistence in Caribbean coral reefs. PLoS One 10:e0129609
Verrill AE (1900) Additions to the Anthozoa and Hydrozoa of the Bermudas. Trans Conn Acad Arts Sci 10:551–572
Waldock C, Stuart-Smith RD, Edgar GJ, Bird TJ, Bates AE (2019) The shape of abundance distributions across temperature gradients in reef fishes. Ecol Lett 22:685–696
Walton CJ, Hayes NK, Gilliam DS (2018) Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front Mar Sci 5:323
Weil E (2004) Coral reef diseases in the Wider Caribbean. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, Berlin, pp 35–68
West DA (1979) Symbiotic zoanthids (Anthozoa: Cnidaria) of Puerto Rico. Bull Mar Sci 29:253–271
Wörheide G, Erpenbeck D (2007) DNA taxonomy of sponges—progress and perspectives. J Mar Biol Assoc UK 87:1629–1633
Wulff JL (2001) Assessing and monitoring coral reef sponges: why and how? Bull Mar Sci 69:831–846
Yang SY, Bourgeois C, Ashworth CD, Reimer JD (2013) Palythoa zoanthid 'barrens’ in Okinawa: examination of possible environmental causes. Zool Stud 52:39
Zea S (1993) Cover of sponges and other sessile organisms in rocky and coral reef habitats of Santa Marta, Colombian Caribbean Sea. Carib J Sci 29:75–88
Zea S, Henkel TP, Pawlik JR (2014) The sponge guide: a picture guide to Caribbean sponges. www.spongeguide.org
Acknowledgments
Fieldwork at Bonaire was supported by the World Wildlife Fund (WWF) Netherlands Biodiversity Fund, the Treub Maatschappij—Society for the Advancement of Research in the Tropics, and by the Nature of the Netherlands program of Naturalis Biodiversity Center. We thank Stichting Nationale Parken Bonaire (STINAPA), Dutch Caribbean Nature Alliance (DCNA) and Dive Friends (Bonaire) for logistic support. Research permits were granted by Bonaire and Rijkswaterstaat. All members of the Magnificent 7 team are thanked for their underwater support. Two anonymous reviewers and the editor provided comments that greatly improved earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Mark Vermeij
Supplementary Information
Below is the link to the electronic supplementary material.
338_2022_2226_MOESM4_ESM.pdf
Fig. S1 nMDS analyses (Stress = 0.097) of Bonaire zoantharian communities in this study. Colored circles indicate different depths of surveys. (PDF 177 KB)
338_2022_2226_MOESM5_ESM.pdf
Fig. S2 Map of Bonaire showing distribution of 20 m and 30 m zoantharian communities. Red sites correspond to sites in the Red grouping in the SIMPROF analyses of Figure 4, blue sites correspond to sites in the Blue grouping in the same figure. Purple sites include communities from both groupings. Map from OpenStreetMap loaded into QGIS. (PDF 3585 KB)
338_2022_2226_MOESM6_ESM.pdf
Fig. S3 nMDS analyses (stress = 0.162) of Bonaire (circles) and Curaçao (squares) zoantharian communities in this study. Colored circles indicate different depths of surveys. The numbers next to squares indicate multiple surveys overlapping (n = 8 and 32 surveys, accordingly). (PDF 210 KB)
338_2022_2226_MOESM7_ESM.pdf
Fig. S4 nMDS analyses (stress = 0.162) identical to Fig. S3 of Bonaire (circles) and Curaçao (squares) zoantharian communities in this study except that N. amorpha and N. erecta are combined into a single taxon. Colored circles indicate different depths of surveys. The numbers next to squares indicate multiple surveys overlapping (n = 8 and 32 surveys, accordingly). (PDF 105 KB)
Rights and permissions
About this article
Cite this article
Reimer, J.D., Wee, H.B., García-Hernández, J.E. et al. Same but different? Zoantharian assemblages (Anthozoa: Hexacorallia) in Bonaire and Curaçao, southern Caribbean. Coral Reefs 41, 383–396 (2022). https://doi.org/10.1007/s00338-022-02226-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02226-x