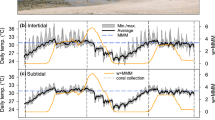
Abstract
Reef-building corals are surrounded by complex microenvironments (i.e. concentration boundary layers) that partially isolate them from the ambient seawater. Although the presence of such concentration boundary layers (CBLs) could potentially play a role in mitigating the negative impacts of climate change stressors, their role is poorly understood. Furthermore, it is largely unknown how heat stress-induced bleaching affects O2 and pH dynamics across the CBLs of coral, particularly in branching species. We experimentally exposed the common coral species Acropora aspera to heat stress for 13 d and conducted a range of physiological and daytime microsensor measurements to determine the effects of bleaching on O2 and pH gradients across the CBL. Heat stress equivalent to 24 degree heating days (3.4 degree heating weeks) resulted in visible bleaching and significant declines in photochemical efficiency, photosynthesis rates and photosynthesis to respiration (P/R) ratios, whereas dark respiration and calcification rates were not impacted. As a consequence, bleached A. aspera had significantly lower (− 13%) surface O2 concentrations during the day than healthy corals, with concentrations being lower than that of the ambient seawater, thus resulting in O2 uptake from the seawater. Furthermore, we show here that Acropora, and potentially branching corals in general, have among the lowest surface pH elevation of all corals studied to date (0.041 units), which could contribute to their higher sensitivity to ocean acidification. Additionally, bleached A. aspera no longer elevated their surface pH above ambient seawater values and, therefore, had essentially no [H+] CBL. These findings demonstrate that heat stress-induced bleaching has negative effects on pH elevation and [H+] CBL thickness, which may increase the overall susceptibility of coral to the combined impacts of ocean acidification and warming.






Similar content being viewed by others
Change history
13 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00338-023-02423-2
References
Agostini S, Fujimura H, Higuchi T, Yuyama I, Casareto BE, Suzuki Y, Nakano Y (2013) The effects of thermal and high-CO2 stresses on the metabolism and surrounding microenvironment of the coral Galaxea fascicularis. Comptes Rendus Biologies 336:384–391
Al-Horani FA (2005) Effects of changing seawater temperature on photosynthesis and calcification in the scleractinian coral Galaxea fascicularis, measured with O2, Ca2+ and pH microsensors. Scientia Marina 69:347–354
Al-Horani FA, Al-Moghrabi SM, De Beer D (2003a) Microsensor study of photosynthesis and calcification in the sceractinian coral, Galaxea fascicularis: active internal carbon cycle. J Exp Mar Biol Ecol 288:1–15
Al-Horani FA, Al-Moghrabi SM, de Beer D (2003b) The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar Biol 142:419–426
Baird A, Marshall P (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Cai W-J, Ma Y, Hopkinson BM, Grottoli AG, Warner ME, Ding Q, Hu X, Yuan X, Schoepf V, Xu H, Han C, Melman TF, Hoadley KD, Pettay DT, Matsui Y, Baumann JH, Levas S, Ying Y, Wang Y (2016) Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat Comm 7:11144
Chan NCS, Wangpraseurt D, Kühl M, Connolly S (2016) Flow and coral morphology control coral surface pH: Implications for the effects of ocean acidification. Frontiers in Marine Science 3:https://doi.org/10.3389/fmars.2016.00010
Cornwall CE, Hepburn CD, Pilditch CA, Hurd CL (2013) Concentration boundary layers around complex assemblages of macroalgae: Implications for the effects of ocean acidification on understory coralline algae. Limnol Oceanogr 58:121–130
Cornwall CE, Boyd PW, McGraw CM, Hepburn CD, Pilditch CA, Morris JN, Smith AM, Hurd CL (2014) Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PLoS One 9:e97235
D’Olivo JP, McCulloch MT (2017) Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci Rep 7:2207
Dandan SS, Falter JL, Lowe RJ, McCulloch MT (2015) Resilience of coral calcification to extreme temperature variations in the Kimberley region, northwest Australia. Coral Reefs 34:1151–1163
de Beer D, Kühl M, Stambler N, Vaki L (2000) A microsensor study of light enhanced Ca2+ uptake and photosynthesis in the reef-building hermatypic coral Favia sp. Mar Ecol Prog Ser 194:75–85
Dickson AG, Sabine CL, Christian JR (2007) Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3:191 pp
Edmunds PJ, Brown D, Moriarty V (2012) Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Global Change Biol 18:2173–2183
Enríquez S, Méndez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50:1025–1032
Garcia HE, Gordon LI (1992) Oxygen solubility in seawater: Better fitting equations. Limnol Oceanogr 37:1307–1312
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck R, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge T, Claar DC, Eakin CAM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom MO, Lowe RJ, McCulloch M, Pandolfi JM, Pratchett MS, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, C-y Kuo, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hurd CL (2015) Slow-flow habitats as refugia for coastal calcifiers from ocean acidification. J Phycol 51:599–605
Hurd CL, Cornwall CE, Currie K, Hepburn CD, McGraw CM, Hunter KA, Boyd PW (2011) Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Global Change Biol 17:3254–3262
IPCC (2013) Climate Change 2013: The physical science basis. Summary for Policy Makers., http://www.ipcc.ch website
Jamieson D, Chance B, Cadenas E, Boveris A (1986) The relation of free radical production to hyperoxia. Annual Review of Physiology 48:703–719
Jimenez IM, Kühl M, Larkum AWD, Ralph PJ (2011) Effects of flow and colony morphology on the thermal boundary layer of corals. J R Soc Interface 8:1785–1795
Jokiel PL, Maragos JE, Franzisket L (1978) Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE (eds) Coral Reefs: Resesarch Methods. UNESCO, Paris, pp 529–541
Jorgensen BB, Revsbech NP (1985) Diffusive boundary layers and the oxygen uptake of sediments and detritus. Limnol Oceanogr 30:112–122
Koren K, Jakobsen SL, Kühl M (2016) In-vivo imaging of O2 dynamics on coral surfaces spray-painted with sensor nanoparticles. Sensors and Actuators B: Chemical 237:1095–1101
Kühl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and Light. Mar Ecol Prog Ser 117:159–172
Legendre P, Legendre L (1998) Numerical Ecology. Elsevier
Maynard JA, Turner PJ, Anthony KRN, Baird AH, Berkelmans R, Eakin CM, Johnson J, Marshall PA, Packer GR, Rea A, Willis BL (2008) ReefTemp: An interactive monitoring system for coral bleaching using high-resolution SST and improved stress predictors. Geophys Res Lett 35:L05603
McCulloch MT, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change 2:623–627
Nishihara GN, Ackerman JD (2007) On the determination of mass transfer in a concentration boundary layer. Limnol Oceanogr Meth 5:88–96
NOAA (2016) NOAA Coral Reef Watch Climatology version 2 NOAA Coral Reef Watch: Methodology
Noisette F, Hurd C (2018) Abiotic and biotic interactions in the diffusive boundary layer of kelp blades create a potential refuge from ocean acidification. Funct Ecol 32:1329–1342
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr 52:1874–1882
Schoepf V, Grottoli AG, Warner M, Cai W-J, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H, Wang Y, Matsui Y, Baumann J (2013) Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS One 8:e75049
Schoepf V, McCulloch MT, Warner ME, Levas SJ, Matsui Y, Aschaffenburg M, Grottoli AG (2014) Short-term coral bleaching is not recorded by skeletal boron isotopes. PLoS One 9:e112011
Schoepf V, Stat M, Falter JL, McCulloch MT (2015a) Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci Rep 5:17639
Schoepf V, Grottoli AG, Levas SJ, Aschaffenburg MD, Baumann JH, Matsui Y, Warner ME (2015b) Annual coral bleaching and the long-term recovery capacity of coral. Proc R Soc B 282:20151887
Schoepf V, Jury CP, Toonen RJ, McCulloch MT (2017) Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc R Soc B: Biol Sci 284:20172117
Shashar N, Cohen Y, Loya Y (1993) Extreme diel fluctuations of oxygen in diffusive boundary layers surrounding stony corals. Biol Bull 185:455–461
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25:453–460
Towle EK, Enochs IC, Langdon C (2015) Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS One 10:e0123394
Venn AA, Tambutte E, Holcomb M, Laurent J, Allemand D, Tambutte S (2013) Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc Natl Acad Sci 110:1634–1639
Wangpraseurt D, Larkum AWD, Ralph PJ, Kühl M (2012) Light gradients and optical microniches in coral tissues. Front Microbiol 3:https://doi.org/10.3389/fmicb.2012.00316
Acknowledgements
Funding for this study was provided by the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies, the Western Australian Marine Science Institution (WAMSI), an ARC Laureate Fellowship awarded to MM and an ARC DECRA Award (DE160100668) awarded to SC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Simon Davy
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schoepf, V., Cornwall, C.E., Pfeifer, S.M. et al. Impacts of coral bleaching on pH and oxygen gradients across the coral concentration boundary layer: a microsensor study. Coral Reefs 37, 1169–1180 (2018). https://doi.org/10.1007/s00338-018-1726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1726-6