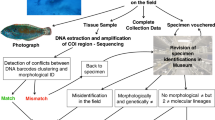
Abstract
DNA Barcoding (DBC) is a method for taxonomic identification of animals that is based entirely on the 5′ portion of the mitochondrial gene, cytochrome oxidase subunit I (COI-5). It can be especially useful for identification of larval forms or incomplete specimens lacking diagnostic morphological characters. DBC can also facilitate the discovery of species and in defining “molecular taxonomic units” in problematic groups. However, DBC is not a panacea for coral reef taxonomy. In two of the most ecologically important groups on coral reefs, the Anthozoa and Porifera, COI-5 sequences have diverged too little to be diagnostic for all species. Other problems for DBC include paraphyly in mitochondrial gene trees and lack of differentiation between hybrids and their maternal ancestors. DBC also depends on the availability of databases of COI-5 sequences, which are still in early stages of development. A global effort to barcode all fish species has demonstrated the importance of large-scale coordination and is yielding promising results. Whether or not COI-5 by itself is sufficient for species assignments has become a contentious question; it is generally advantageous to use sequences from multiple loci.


Similar content being viewed by others
References
Angelov S, Harb B, Kannan S, Khanna S, Kim J, Wang LS (2004) Genome identification and classification by short oligo arrays. In: Jonassen I, Kim J (eds) Algorithms in Bioinformatics, 4th International Workshop, WABI 2004, Bergen, Norway, September 17–21, 2004, Proceedings. Lecture Notes in Computer Science Vol 3240. Springer, Berlin, pp 400–411
Avise JC (1994) Molecular markers, natural history and evolution. Chapman and Hall, New York
Avise JC, Ball RM (1990) Principles of genealogical concordance in species concepts and biological taxonomy. Oxf Surv Evol Biol 7:45–67
Baldwin BS, Black M, Sanjur O, Gustafson R, Lutz RA, Vrijenhoek RC (1996) A diagnostic molecular marker for zebra mussels (Dreissena polymorpha) and potentially co-occurring bivalves: Mitochondrial COI. Mol Mar Biol Biotechnol 5:9–14
Barber P, Boyce SL (2006) Estimating diversity of Indo-Pacific coral reef stomatopods through DNA barcoding of stomatopod larvae. Proc R Soc Lond B Biol Sci 273:2053–2061
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bhadury P, Austen MC, Bilton DT, Lambshead PJD, Rogers AD, Smerdon GR (2006) Development and evaluation of a DNA-barcoding approach for the rapid identification of nematodes. Mar Ecol Prog Ser 320:1–9
Bilodeau AL, Lankford WS, Kim TJ, Felder DL, Neigel JE (1999) An ultrasensitive method for detection of single crab larvae (Sesarma reticulatum) using PCR amplification of a highly repetitive DNA sequence. Mol Ecol 8:683–684
Blaxter ML (2004) The promise of a DNA taxonomy. Proc R Soc Lond B Biol Sci 359:669–679
Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E (2005) Defining operational taxonomic units using DNA barcode data. Proc R Soc Lond B Biol Sci 360:1935–1943
Borneman J (2001) Probe selection algorithms with applications in the analysis of microbial communities. Bioinformatics 17:1–9
Brower AVZ (2006) Problems with DNA barcodes for species delimitation: ‘ten species’ of Astraptes fulgerator reassessed (Lepidoptera : Hesperiidae). Systematics and Biodiversity 4:127–132
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1996) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
Call DR (2005) Challenges and opportunities for pathogen detection using DNA microarrays. Crit Rev Microbiol 31:91–99
Cognato AI (2006) Standard percent DNA sequence difference for insects does not predict species boundaries. J Econ Entomol 99:1037–1045
Collins LS, Budd AF, Coates AG (1996) Earliest evolution associated with closure of the Tropical American Seaway. Proc Natl Acad Sci USA 93:6069–6072
Cote IM, Gill JA, Gardner TA, Watkinson AR (2005) Measuring coral reef decline through meta-analyses. Proc R Soc Lond B Biol Sci 360:385–395
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
DeLong EE (2005) Microbial community genomics in the ocean. Nat Rev Microbiol 3:459–469
Edwards RA, Rodriguez-Brito B, Wegley L, Haynes M, Breitbart M, Peterson DM, Saar MO, Alexander S, Alexander EC, Rohwer F (2006) Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics 7:57
Erpenbeck D, Hooper JNA, Worheide G (2006) CO1 phylogenies in diploblasts and the ‘Barcoding of Life’—are we sequencing a suboptimal partition? Mol Ecol Notes 6:550–553
Floyd R, Abebe E, Papert A, Blaxter M (2002) Molecular barcodes for soil nematode identification. Mol Ecol 11:839–850
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Froese R, Pauly D (2000) FishBase 2000: concepts, design and data sources. ICLARM, Los Baños
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: Frequency, causes, and consequences with insights from animal mitochondrial DNA. Annual Review of Ecology, Evolution and Systematics 34:397–423
Gaines SD, Gaylord B, Largier JL (2003) Avoiding current oversights in marine reserve design. Ecol Appl 13:S32–S46
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Gaylord B, Gaines SD, Siegel DA, Carr MH (2005) Marine reserves exploit population structure and life history in potentially improving fisheries yields. Ecol Appl 15:2180–2191
Georgiev GP, Kramerov DA, Ryskov AP, Skryabin KG, Lukanidin EM (1982) Dispersed repetitive sequences in eukaryotic genomes and their possible biological significance. Cold Spring Harbor Symp Quant Biol 47:1109–1121
Goffredi SK, Jones WJ, Scholin CA, Marin R, Vrijenhoek RC (2006) Molecular detection of marine invertebrate larvae. Mar Biotechnol 8:149–160
Gómez A, Wright PJ, Lunt DH, Cancino JM, Carvalho GR, Hughes RN (2007) Mating trials validate the use of DNA barcoding to reveal cryptic speciation of a marine bryozoan taxon. Proc R Soc Lond B Biol Sci 274:199–207
Govindarajan AF, Halanych KK, Cunningham CW (2005) Mitochondrial evolution and phylogeography in the hydrozoan Obelia geniculata (Cnidaria). Mar Biol 146:213–222
Grantham BA, Eckert GL, Shanks AL (2003) Dispersal potential of marine invertebrates in diverse habitats. Ecol Appl 13:S108–S116
Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PDN (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proc Natl Acad Sci USA 103:968–971
Hanner R (2005) Proposed Standards for BARCODE Records in INSSC (BRIs) Database Working Group, Consortium for the Barcode of Life, http://www.barcoding.si.edu/
Hare MP, Palumbi SR, Butman CA (2000) Single-step species identification of bivalve larvae using multiplex polymerase chain reaction. Mar Biol 137:953–961
Hebert PDN, Ratnasingham S, deWaard JR (2003a) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B Biol Sci 270:S96–S99
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003b) Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci 270:313–321
Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004a) Identification of birds through DNA barcodes. PLoS Biology 2:1657–1663
Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W (2004b) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA 101:14812–14817
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Hellberg ME (2006) No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evol Biol 6:24
Holland BS, Dawson MN, Crow GL, Hofmann DK (2004) Global phylogeography of Cassiopea (Scyphozoa : Rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar Biol 145:1119–1128
Janzen DH (2004) Now is the time. Proc R Soc Lond B Biol Sci 359:731–732
Johnson NK, Cicero C (2004) New mitochondrial DNA data affirm the importance of Pleistocene speciation in North American birds. Evolution 58:1122–1130
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci USA 101:8251–8253
Knowlton N (1993) Sibling Species in the Sea. Annu Rev Ecol Syst 24:189–216
Knowlton N (2001) The future of coral reefs. Proc Natl Acad Sci USA 98:5419–5425
Lazoski C, Sole-Cava AM, Boury-Esnault N, Klautau M, Russo CAM (2001) Cryptic speciation in a high gene flow scenario in the oviparous marine sponge Chondrosia reniformis. Mar Biol 139:421–429
Lefebure T, Douady CJ, Gouy M, Gibert J (2006) Relationship between morphological taxonomy and molecular divergence within Crustacea: Proposal of a molecular threshold to help species delimitation. Mol Phylogenet Evol 40:435–447
Lipscomb D, Platnick N, Wheeler Q (2003) The intellectual content of taxonomy: a comment on DNA taxonomy. Trends Ecol Evol 18:65–66
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5 ‘ nuclease assay. Genet Anal Biomol Eng 14:143–149
Livak KJ, Flood SJA, Marmaro J, Giusti W, Deetz K (1995) Oligonucleotides with fluorescent dyes at oppposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods & Applications 4:357–362
Mace GM (2004) The role of taxonomy in species conservation. Philos Trans R Soc Lond B 359:711–719
MaKinster JG, Roberts JE, Felder DL, Chlan CA, Bourdreaux M, Bilodeau AL, Neigel JE (1999) PCR amplification of a middle repetitive element detects larval stone crabs (Crustacea: Decapoda: Menippidae) in estuarine plankton samples. Mar Ecol Prog Ser 188:161–168
Markmann M, Tautz D (2005) Reverse taxonomy: an approach towards determining the diversity of meiobenthic organisms based on ribosomal RNA signature sequences. Proc R Soc Lond B Biol Sci 360:1917–1924
Martin AP, Palumbi SR (1993) Body size, metabolic-rate, generation time, and the molecular clock. Proc Natl Acad Sci USA 90:4087–4091
Matz MV, Nielsen R (2005) A likelihood ratio test for species membership based on DNA sequence data. Proc R Soc Lond B Biol Sci 360:1969–1974
McClanahan TR (2002) The near future of coral reefs. Environ Conserv 29:460–483
McFadden CS, Tullis I, Hutchinson MB, Winner K (2000) Rates of evolution of cnidarian mitochondrial genes. Am Zool 40:1124–1124
McManus J (1985) Marine speciation, tectonics and sea-level changes in southeast Asia. Proc 5th Int Coral Reef Congr 4:133–138
Meyer CP, Paulay G (2005) DNA barcoding: Error rates based on comprehensive sampling. PLoS Biology 3:2229–2238
Mikkelsen PM, Cracraft J (2001) Marine biodiversity and the need for systematic inventories. Bull Mar Sci 69:525–534
Moritz C, Cicero C (2004) DNA barcoding: Promise and pitfalls. PLoS Biology 2:1529–1531
Naviaux RK, Good B, McPherson JD, Steffen DL, Markusic D, Ransom B, Corbeil J (2005) Sand DNA - a genetic library of life at the water’s edge. Mar Ecol Prog Ser 301:9–22
Neigel JE (2003) Species-area relationships and marine conservation. Ecol Appl 13:S138–S145
Neigel JE, Avise JC (1986) Phylogenetic relationships of mitochondrial DNA under various demographic models of speciation. In: Karlin S, Nevo E (eds) Evolutionary Processes and Theory. Academic Press, New York, pp 515–534
Nielsen R, Matz M (2006) Statistical approaches for DNA barcoding. Syst Biol 55:162–169
O’Dor R, Gallardo VA (2005) How to census marine life: ocean realm field projects. Sci Mar 69:181–199
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Paquin P, Hedin M (2004) The power and perils of ‘molecular taxonomy’: a case study of eyeless and endangered Cicurina (Araneae : Dictynidae) from Texas caves. Mol Ecol 13:3239–3255
Pegg G, Sinclair B, Briskey L, Aspden W (2006) MtDNA barcode identification of fish larvae in the southern Great Barrier Reef, Australia. Sci Mar 70S2:7–12
Powers T (2004) Nematode molecular diagnostics: From bands to barcodes. Annu Rev Phytopahtol 42:367–383
Ratnasingham S, Hebert PDN (2007) BOLD: The Barcode of Life Data Systems. Mol Ecol Notes 7:355–364
Reaka-Kudla ML (1997) The global biodiversity of coral reefs: A comparison with rain forests. In: Reaka-Kudla ML, Wilson DE, Wilson EO (eds) Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press, Washington, pp 83–108
Reaka-Kudla ML (2000) The evolution of endemism in insular Pacific faunas: coral-dwelling stomatopods. Journal of Crustacean Biology 20:56–70
Relogio A, Schwager C, Richter A, Ansorge W, Valcarcel J (2002) Optimization of oligonucleotide-based DNA microarrays. Nucl Acids Res 30:e51
Richardson D, Vanwye J, Exum A, Cowen R, Crawford D (2006) High-throughput species identification: from DNA isolation to bioinformatics. Mol Ecol Notes “in press”
Robba L, Russell SJ, Barker GL, Brodie J (2006) Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am J Bot 93:1101–1108
Roberts CM (1997) Connectivity and management of Caribbean coral reefs. Science 278:1454–1457
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284
Romano SL, Palumbi SR (1996) Evolution of scleractinian corals inferred from molecular systematics. Science 271:640–642
Rubinoff D (2006) DNA barcoding evolves into the familiar. Conserv Biol 20:1548–1549
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Proc R Soc Lond B Biol Sci 360:1879–1888
Schander C, Willassen E (2005) What can biological barcoding do for marine biology?. Mar Biol Res 1:79–83
Shaw KL (2002) Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: What mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. Proc Natl Acad Sci USA 99:16122–16127
Shearer TL, Coffroth MA (2006) Genetic identification of Caribbean scleractinian coral recruits at the Flower Garden Banks and the Florida Keys. Mar Ecol Prog Ser 306:133–142
Shearer TL, Van Oppen MJH, Romano SL, Worheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Smith PJ, McVeagh SM, Allain V, Sanchez C (2005) DNA identification of gut contents of large pelagic fishes. J Fish Biol 67:1178–1183
St Mary CM, Osenberg CW, Frazer TK, Lindberg WJ (2000) Stage structure, density dependence and the efficacy of marine reserves. Bull Mar Sci 66:675–690
Stanley SM (1998) Macroevolution, Pattern and Process. Johns Hopkins University Press, Baltimore
Summerbell RC, Levesque CA, Seifert KA, Bovers M, Fell JW, Diaz MR, Boekhout T, de Hoog GS, Stalpers J, Crous PW (2005) Microcoding: the second step in DNA barcoding. Proc R Soc Lond B Biol Sci 360:1897–1903
Tringe SG, Rubin EM (2005) Metagenomics: DNA sequencing of environmental samples. Nat Rev Genet 6:805–814
Vadopalas B, Bouma JV, Jackels CR, Friedman CS (2006) Application of real-time PCR for simultaneous identification and quantification of larval abalone. J Exp Mar Biol Ecol 334:219–228
van Oppen MJH, Gates RD (2006) Conservation genetics and the resilience of reef-building corals. Mol Ecol 15:3863–3883
Vawter L, Brown WM (1986) Nuclear and mitochondrial DNA comparisons reveal extreme rate variation in the molecular clock. Science 234:194–196
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu DY, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Proc R Soc Lond B Biol Sci 360:1847–1857
Watanabe S, Minegishi Y, Yoshinaga T, Aoyama J, Tsukamoto K (2004) A quick method for species identification of Japanese eel (Anguilla japonica) using real-time PCR: An onboard application for use during sampling surveys. Mar Biotechnol 6:566–574
Webb KE, Barnes DKA, Clark MS, Bowden DA (2006) DNA barcoding: A molecular tool to identify Antarctic marine larvae. Deep-Sea Res Part II 53:1053–1060
Wheeler QD (2005) Losing the plot: DNA “barcodes” and taxonomy. Cladistics 21:405–407
Will KW, Rubinoff D (2004) Myth of the molecule: DNA barcodes for species cannot replace morphology for identification and classification. Cladistics 20:47–55
Will KW, Mishler BD, Wheeler QD (2005) The perils of DNA barcoding and the need for integrative taxonomy. Syst Biol :54
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Acknowledgments
We thank Bob Hanner for allowing us to analyze the progress of FISH-BOL and for providing us with updates on CBOL projects, James Albert and Emily Capuli for providing us with a current list of reef-associated fish species, Bob Vrijenhoek and two anonymous reviewers for helpful comments, and the National Science Foundation for support (OCE 0326383). We also thank the organizers and participants of the Coral Reef Barcoding and Environmental Sampling Workshop held at the Smithsonian Tropical Research Institute in 2005 for their stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor M. van Oppen.
Rights and permissions
About this article
Cite this article
Neigel, J., Domingo, A. & Stake, J. DNA barcoding as a tool for coral reef conservation. Coral Reefs 26, 487–499 (2007). https://doi.org/10.1007/s00338-007-0248-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-007-0248-4