Abstract
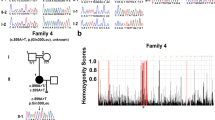
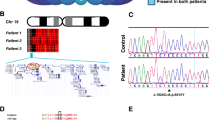
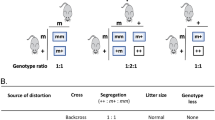
An N-ethyl-N-nitrosourea random mutation screen was used to identify recessive modifiers of gene silencing in the mouse using an epigenetically sensitive reporter transgene. One of the mutant lines, MommeR1, was identified as a suppressor of variegation and it showed female-specific age-associated infertility in homozygotes. Linkage analysis identified a region on chromosome 10, containing the Foxo3a gene, previously shown to play a critical role in female gametogenesis. Foxo3a is a transcription factor with roles in cell cycle control, apoptosis, neural and hematopoietic cell differentiation, and DNA repair. Sequencing of the Foxo3a gene in MommeR1 mice revealed a point mutation that causes an amino acid substitution in the highly conserved Forkhead DNA-binding domain. In vitro transcription assays showed that the point mutation causes loss of FOXO3a transactivation activity. Compound heterozygotes made with Foxo3a-null mice (carrying the targeted deletion of exon 2) displayed complementation with respect to both the activation of the reporter transgene and defects in folliculogenesis similar to those seen in MommeR1 homozygotes, supporting the conclusion that this is the causative mutation. Approximately one in six female MommeR1 homozygotes develop teratomas, a phenotype not reported in Foxo3a-null mice. Ovulated oocytes from MommeR1 homozygotes display a number of abnormalities. The MommeR1 mice provide a novel platform to investigate teratocarcinogenesis and link Foxo3a with parthenogenesis and ovarian cancer. The finding of Foxo3a as a modifier of epigenetic reprogramming is discussed.






Similar content being viewed by others
References
Ashe A, Morgan DK, Whitelaw NC, Bruxner TJ, Vickaryous NK, Cox LL, Butterfield NC, Wicking C, Blewitt ME, Wilkins SJ, Anderson GJ, Cox TC, Whitelaw E (2008) A genome-wide screen for modifiers of transgene variegation identifies genes with critical roles in development. Genome Biol 9:R182
Bakker WJ, Blazquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, Coffer PJ, Lowenberg B, von Lindern M, van Dijk TB (2004) FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol 164:175–184
Banerjee-Basu S, Baxevanis AD (2004) Structural analysis of disease-causing mutations in the P-subfamily of forkhead transcription factors. Proteins 54:639–647
Blewitt ME, Vickaryous NK, Hemley SJ, Ashe A, Bruxner TJ, Preis JI, Arkell R, Whitelaw E (2005) An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc Natl Acad Sci USA 102:7629–7634
Blewitt ME, Vickaryous NK, Paldi A, Koseki H, Whitelaw E (2006) Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet 2:e49
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868
Carlsson P, Mahlapuu M (2002) Forkhead transcription factors: key players in development and metabolism. Dev Biol 250:1–23
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218
Chong S, Vickaryous N, Ashe A, Zamudio N, Youngson N, Hemley S, Stopka T, Skoultchi A, Matthews J, Scott HS, de Kretser D, O’Bryan M, Blewitt M, Whitelaw E (2007) Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat Genet 39:614–622
Collins JJ, Montali RJ, Manus AG (1987) Toxicological evaluation of 4-vinylcyclohexene. II. Induction of ovarian tumors in female B6C3F1 mice by chronic oral administration of 4-vinylcyclohexene. J Toxicol Environ Health 21:507–524
Eppig JJ, Wigglesworth K, Varnum DS, Nadeau JH (1996) Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer Res 56:5047–5054
Fafalios MK, Olander EA, Melhem MF, Chaillet JR (1996) Ovarian teratomas associated with the insertion of an imprinted transgene. Mamm Genome 7:188–193
Fei M, Zhao Y, Wang Y, Lu M, Cheng C, Huang X, Zhang D, Lu J, He S, Shen A (2009) Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest 27:52–59
Furuyama T, Nakazawa T, Nakano I, Mori N (2000) Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349:629–634
Furuyama T, Banerjee R, Breen TR, Harte PJ (2004) SIR2 is required for polycomb silencing and is associated with an E(Z) histone methyltransferase complex. Curr Biol 14:1812–1821
Guarente L (2000) Sir2 links chromatin silencing, metabolism, and aging. Genes Dev 14:1021–1026
Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y et al (1994) Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370:68–71
Hogan B, Beddington R, Costantini F, Lacy E (1994) Manipulating the mouse embryo, a laboratory manual. Cold Spring Laboratory Press, Cold Spring Harbor, NY
Hsu SY, Lai RJ, Finegold M, Hsueh AJ (1996) Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 137:4837–4843
Kaestner KH, Knochel W, Martinez DE (2000) Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev 14:142–146
Kimura N, Tsunoda S, Iuchi Y, Abe H, Totsukawa K, Fujii J (2010) Intrinsic oxidative stress causes either 2-cell arrest or cell death depending on developmental stage of the embryos from SOD1-deficient mice. Mol Hum Reprod 16:441–451
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321
Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP (2001) A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413:519–523
Li M, Chiu JF, Mossman BT, Fukagawa NK (2006) Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem 281:40429–40439
Liu H, Luo LL, Qian YS, Fu YC, Sui XX, Geng YJ, Huang DN, Gao ST, Zhang RL (2009) FOXO3a is involved in the apoptosis of naked oocytes and oocytes of primordial follicles from neonatal rat ovaries. Biochem Biophys Res Commun 381:722–727
Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K (2007) Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 134:199–209
Matrone A, Grossi V, Chiacchiera F, Fina E, Cappellari M, Caringella AM, Di Naro E, Loverro G, Simone C (2010) p38alpha is required for ovarian cancer cell metabolism and survival. Int J Gynecol Cancer 20:203–211
Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T (2008) FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood 112:4485–4493
Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB (2004) Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127:569–580
Nemoto S, Fergusson MM, Finkel T (2004) Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306:2105–2108
Ormestad M, Blixt A, Churchill A, Martinsson T, Enerback S, Carlsson P (2002) Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci 43:1350–1357
Preis JI, Downes M, Oates NA, Rasko JE, Whitelaw E (2003) Sensitive flow cytometric analysis reveals a novel type of parent-of-origin effect in the mouse genome. Curr Biol 13:955–959
Saleem RA, Banerjee-Basu S, Berry FB, Baxevanis AD, Walter MA (2001) Analyses of the effects that disease-causing missense mutations have on the structure and function of the winged-helix protein FOXC1. Am J Hum Genet 68:627–641
Saleem RA, Banerjee-Basu S, Berry FB, Baxevanis AD, Walter MA (2003) Structural and functional analyses of disease-causing missense mutations in the forkhead domain of FOXC1. Hum Mol Genet 12:2993–3005
Stevens LC, Varnum DS (1974) The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev Biol 37:369–380
Strodicke M, Karberg S, Korge G (2000) Domina (Dom), a new Drosophila member of the FKH/WH gene family, affects morphogenesis and is a suppressor of position-effect variegation. Mech Dev 96:67–78
Sui XX, Luo LL, Xu JJ, Fu YC (2010) Evidence that FOXO3a is involved in oocyte apoptosis in the neonatal rat ovary. Biochem Cell Biol 88:621–628
Tan WQ, Wang K, Lv DY, Li PF (2008) Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem 283:29730–29739
van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM (2004) FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1). J Biol Chem 279:28873–28879
Weigelt J, Climent I, Dahlman-Wright K, Wikstrom M (2001) Solution structure of the DNA binding domain of the human forkhead transcription factor AFX (FOXO4). Biochemistry 40:5861–5869
Acknowledgments
The authors thank Graham Kay for technical advice and equipment for culturing oocytes and Ronald DePinho for the use of the Foxo3a null mutant mice. This study was supported by Australian NHMRC project grants to EW. EW is supported by an NHMRC Australia Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. A. Youngson and N. Vickaryous are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youngson, N.A., Vickaryous, N., van der Horst, A. et al. A missense mutation in the transcription factor Foxo3a causes teratomas and oocyte abnormalities in mice. Mamm Genome 22, 235–248 (2011). https://doi.org/10.1007/s00335-011-9317-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-011-9317-7