Abstract
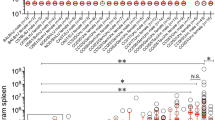
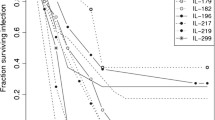
EUMORPHIA (European Union Mouse Research for Public Health and Industrial Application) is a research program involved in developing new approaches in phenotyping, mutagenesis, and informatics to improve characterization of mouse models for understanding human physiology and disease. Secondary screen experiments include the development of assays to identify mice with altered susceptibility or resistance to infections. In this context we developed a new model and established a standard operating procedure for the experimental infection of mice with Yersinia (Y.) enterocolitica. In contrast with previous studies that dealt with high-pathogenic Y. enterocolitica, we used the low-pathogenic Y. enterocolitica strain E40 to analyze differences in the immune response of four strains of inbred mice (BALB/c, C3H/HeN, 129P2, C57BL/6) after oral infection. The determination of colony-forming units in Peyer’s patches and histologic analysis supported the observations that BALB/c are less able to ameliorate the infection within 21 days. The immune defense of C57BL/6 mice against Yersinia was the most effective resulting in a nearly complete elimination of bacteria after 21 days. C3H/HeN and 129P2 were intermediate. Analysis of serum immunoglobulins (Ig) by Luminex showed a significant increase of IgG2b levels 21 days after infection in all four inbred strains. The other immunoglobulins remained nearly constant. Our infection model discriminates between the efficiency of an infection at an early time point (3 days) and immunity at a later time point (21 days). It is furthermore an appropriate model to characterize genetic differences in resistance and immunity of inbred and mutant mouse lines.




Similar content being viewed by others
References
Alexander AD, Orcutt RP, Henry JC, Baker J Jr, Bissahoyo AC et al (2006) Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome 14:1093–1104
Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB (1983) Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 258:7155–7160
Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK et al (1987) Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA 84:6020–6024
Autenrieth IB, Heesemann J (1992) In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol 181:333–338
Autenrieth IB, Beer M, Hantschmann P, Preger S, Vogel U et al (1993) The cellular immune response against Yersinia enterocolitica in different inbred strains of mice: evidence for an important role of T lymphocytes. Zentralbl Bakteriol 278:383–395
Autenrieth IB, Beer M, Bohn E, Kaufmann SH, Heesemann J (1994) Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun 62:2590–2599
Autenrieth IB, Kempf V, Sprinz T, Preger S, Schnell A (1996) Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun 64:1357–1368
Bakour R, Balligand G, Laroche Y, Cornelis G, Wauters G (1985) A simple adult-mouse test for tissue invasiveness in Yersinia enterocolitica strains of low experimental virulence. J Med Microbiol 19:237–246
Bohn E, Autenrieth IB (1996) IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol 156:1458–1468
Bohn E, Schmitt E, Bielfeldt C, Noll A, Schulte R et al (1998) Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect Immun 66:2213–2220
Bottone EJ (1997) Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev 10:257–276
Cangiani EE, Guiraldi FR, Pinto SE, de Medeiros BM (2007) Kinetics of B cell response during Yersinia enterocolitica infection in resistant and susceptible strains of mice. Immunol Invest 36:387–402
Carniel E, Guilvout I, Prentice M (1996) Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J Bacteriol 178:6743–6751
Carter PB (1975) Pathogenicity of Yersinia enterocolitica for mice. Infect Immun 11:164–170
de Medeiros BM, de Moraes Costa A, de Araújo PM, Falcão DP (1995) Occurrence of polyclonal B-cell activation after experimental infection with Yersinia enterocolitica O:3. Contrib Microbiol Immunol 13:207–210
Foultier B, Troisfontaines P, Muller S, Opperdoes FR, Cornelis GR (2002) Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogeny analysis of type III secretion systems. J Mol Evol 55:37–51
Gemski P, Lazere JR, Casey T (1980) Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun 27:682–685
Grutzkau A, Hanski C, Hahn H, Riecken EO (1990) Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011–1015
Haller JC, Carlson S, Pederson KJ, Pierson DE (2000) A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol Microbiol 36:1436–1446
Hancock GE, Schaedler RW, MacDonald TT (1986) Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect Immun 53:26–31
Hancock GE, Schaedler RW, MacDonald TT (1988) Multigenic control of resistance to Yersinia enterocolitica in inbred strains of mice. Infect Immun 56:532–533
Handley SA, Dube PH, Revell PA, Miller VL (2004) Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect Immun 72:1645–1656
Handley SA, Dube PH, Miller VL (2006) Histamine signaling through the H(2) receptor in the Peyer’s patch is important for controlling Yersinia enterocolitica infection. Proc Natl Acad Sci USA 103:9268–9273
Hanski C, Kutschka U, Schmoranzer HP, Naumann M, Stallmach A et al (1989) Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun 57:673–678
Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB et al (1986a) Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol 137:3855–3860
Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M et al (1986b) Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med 163:1037–1050
Köhler H (ed) (1982) Unspezifische innere Krankheitsbedingungen, 9th ed. (Stuttgart: T. Kitt, Ferdinand Enke Verlag)
Kono DH, Ogasawara M, Yu DT (1985) An animal model to study serum immunoglobulin response to infection by Yersinia enterocolitica. Clin Exp Rheumatol 3:117–121
McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR et al (1993) Transforming growth factor beta 1 selectively stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med 177:1031–1037
Portnoy DA, Moseley SL, Falkow S (1981) Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun 31:775–782
Ruiz-Bravo A, Moreno E, Jimenez-Valera M (2001) Intestinal infection of BALB/c mice with Yersinia enterocolitica O9 causes major modifications in phenotype and functions of spleen cells. Microbiology 147:3165–3169
Russel WMS, Burch RL (eds) (1959) The principles of humane experimental technique. Methuen, London
Sory MP, Boland A, Lambermont I, Cornelis GR (1995) Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA 92:11998–12002
van Erp K, Dach K, Koch I, Heesemann J, Hoffmann R (2006) Role of strain differences on host resistance and the transcriptional response of macrophages to infection with Yersinia enterocolitica. Physiol Genomics 25:75–84
Wauters G, Kandolo K, Janssens M (1987) Revised biogrouping scheme of Yersinia enterocolitica. Contrib Microbiol Immunol 9:14–21
Zink DL, Feeley JC, Wells JG, Vanderzant C, Vickery JC et al (1980) Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature 283:224–226
Acknowledgments
The authors are grateful to Guy R. Cornelis (Focal Area Infection Biology, University of Basel, Switzerland) for providing Y. enterocolitica E40. Many thanks to Sieglinde Keilholtz-Gast and Mirjam Schwarzkopf for technical assistance and to Marina Greweling for technical assistance and discussion. This work was supported by EUMORPHIA (European Union Mouse Research for Public Health and Industrial Application).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schippers, A., Mateika, S., Prochnow, B. et al. Susceptibility of four inbred mouse strains to a low-pathogenic isolate of Yersinia enterocolitica . Mamm Genome 19, 279–291 (2008). https://doi.org/10.1007/s00335-008-9105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-008-9105-1