Abstract
Background
Personalising management of primary oesophageal adenocarcinoma requires better risk stratification. Lack of independent validation of proposed imaging biomarkers has hampered clinical translation. We aimed to prospectively validate previously identified prognostic grey-level co-occurrence matrix (GLCM) CT features for 3-year overall survival.
Methods
Following ethical approval, clinical and contrast-enhanced CT data were acquired from participants from five institutions. Data from three institutions were used for training and two for testing. Survival classifiers were modelled on prespecified variables (‘Clinical’ model: age, clinical T-stage, clinical N-stage; ‘ClinVol’ model: clinical features + CT tumour volume; ‘ClinRad’ model: ClinVol features + GLCM_Correlation and GLCM_Contrast). To reflect current clinical practice, baseline stage was also modelled as a univariate predictor (‘Stage’). Discrimination was assessed by area under the receiver operating curve (AUC) analysis; calibration by Brier scores; and clinical relevance by thresholding risk scores to achieve 90% sensitivity for 3-year mortality.
Results
A total of 162 participants were included (144 male; median 67 years [IQR 59, 72]; training, 95 participants; testing, 67 participants). Median survival was 998 days [IQR 486, 1594]. The ClinRad model yielded the greatest test discrimination (AUC, 0.68 [95% CI 0.54, 0.81]) that outperformed Stage (ΔAUC, 0.12 [95% CI 0.01, 0.23]; p = .04). The Clinical and ClinVol models yielded comparable test discrimination (AUC, 0.66 [95% CI 0.51, 0.80] vs. 0.65 [95% CI 0.50, 0.79]; p > .05). Test sensitivity of 90% was achieved by ClinRad and Stage models only.
Conclusions
Compared to Stage, multivariable models of prespecified clinical and radiomic variables yielded improved prediction of 3-year overall survival.
Clinical relevance statement
Previously identified radiomic features are prognostic but may not substantially improve risk stratification on their own.
Key Points
• Better risk stratification is needed in primary oesophageal cancer to personalise management.
• Previously identified CT features—GLCM_Correlation and GLCM_Contrast—contain incremental prognostic information to age and clinical stage.
• Compared to staging, multivariable clinicoradiomic models improve discrimination of 3-year overall survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oesophageal cancer presents a major burden worldwide [1]. A multimodal treatment approach (surgery with chemotherapy or chemoradiotherapy) is standard of care following landmark trials [2,3,4] and offers the best chance of survival for resectable cancer. However, despite this, outcome remains poor for patients treated with curative intent with mortality rates of 45–53% in the first year post diagnosis [5] and median post-progression survival of only 13 months [6]. There is a greater need for personalisation of management to obviate treatment in patients who may not benefit substantially. Alongside this, with the growing interest in total neoadjuvant therapy, and ongoing trials of perioperative immunotherapy, better initial risk stratification of patients at diagnosis is needed to guide management.
Currently, the clinical TNM (tumour-node-metastasis) stage guides management [7] but it has a low predictive accuracy with contemporaneous pathological stage for early stage cancers [8], as well as limitations in prognostication. Prognostic information has central importance for patient decision-making, with cancer patients ranking prognosis, diagnosis and treatment options as their highest information priority [9].
Modelling studies have demonstrated scope to improve upon prognostication in oesophageal adenocarcinoma, including through CT imaging radiomic approaches [10,11,12]. Initial publications have highlighted the potential of different locoregional features. For example, Piazzese et al identified an association of CT grey-level zone distance variance with overall survival in a multicentre cohort (n = 213), which was independent of dimensionality and contrast administration [11], whilst Larue et al developed a random forest radiomic model including 40 CT features to predict 3-year overall survival (OS) in oesophageal cancer patients treated with chemoradiotherapy (n = 239) [12].
To date, clinical translation of imaging radiomic models has been hampered by a relative paucity of independent external validation. Thus, we aimed to validate a prognostic model for 3-year OS including prespecified clinical features and previously proposed CT radiomic features in a prospective multicentre setting for patients with primary oesophageal adenocarcinoma planned for curative treatment.
Methods
Participants and datasets
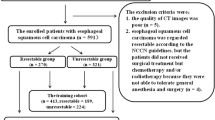
Following ethical approval, clinical data and CT imaging were obtained prospectively from five institutions participating in the OCCAMS (Oesophageal Cancer Clinical and Molecular Stratification) Consortium. Consecutive participants with non-metastatic, pathologically proven oesophageal adenocarcinoma who underwent staging contrast-enhanced CT imaging and planned for definitive treatment were eligible. Participants were excluded if (1) no tumour was visible on CT; (2) CT images were unavailable/corrupted; and (3) concurrent malignancy was present. Data from three institutions were used for model development and two institutions for model testing.
CT imaging and analysis
Contrast-enhanced CT was performed according to institutional practice and included arterial phase imaging of the thorax and upper abdomen. CT acquisition and reconstruction parameters for the training and test datasets are summarised in Supplementary Table 1. Pre-processing of CT images was undertaken as per Image Biomarker Standardisation Initiative recommendations [13]. CT slice thickness was linearly interpolated to 2 mm and attenuation values were converted to Hounsfield units with PyDICOM [14]. The primary tumour was segmented by a radiologist (with 5 years’ experience), who was blinded to clinical outcomes, on the arterial phase thoraco-abdominal CT images. The rationale for using the arterial versus portal venous phase was prior studies showing better tumour conspicuity [15] and tumour staging accuracy [16] for the arterial phase. Tumour segmentations were reviewed and adjusted as required by a second radiologist (with > 20 years’ experience). Examples of tumour segmentations are shown in Supplementary Figs. 1 and 2. 3D Radiomic features were extracted from the segmented volume of interest using PyRadiomics version 3.0.1 under default parameters (no image filters, no normalisation, no voxel array shift, grey-level discretisation at fixed bin width of 25 Hounsfield units) [17].
Radiomic feature selection
Following recommendations [18], radiomic features were pre-selected based on previous published studies. This obviated data-driven feature selection thereby reducing the risk of data overfitting. Published studies were evaluated using the ‘Transparent reporting of a multivariable prediction model for individual prognosis’ (TRIPOD) [19] and ‘Radiomics Quality Score’ checklists [20]. Identifiable Image Biomarker Standardisation Initiative features were sought [13].
Of the identified published studies [11, 12, 21, 22], features proposed by Piazzese et al were excluded as the interpolation strategy employed (2 mm isotropic) would have resulted in a significant reduction of the axial image resolution [11]. Features proposed by Larue et al were excluded as they evaluated nonlinear effects in a high-dimensional feature set using a random forest method, complicating the extraction of a few individually informative linear predictions [12]. Zhang et al achieved a high TRIPOD score of 30 [21] and examined a limited number of previously proposed predictors, each of which was identifiable; thus, these features were extracted—GLCM_Contrast, GLCM_Correlation and GLCM_InverseDifferenceMoment. Furthermore, GLCM correlation was independently identified as a predictor of response in Klaasen et al [22]. Following Peduzzi and Concato’s guideline recommendation of more than 10 events per modelled feature [23], an unsupervised method was applied to identify the most suitable two of the three proposed features. The most collinear GLCM feature with respect to tumour volume and the other GLCM features was excluded.
Statistical analysis
Differences of participant characteristics between training and testing datasets were tested with the Fisher test for categorical variables or the two-sided t-test for continuous variables. For modelling, unpenalised logistic regression models were fitted to predict 3-year OS using base R. With a median survival of 24 months following surgery alone and 46 months following neoadjuvant chemoradiotherapy and surgery, 3-year OS is a meaningful endpoint in oesophageal cancer and has been used in clinical trials assessing the efficacy of neoadjuvant treatment [24].
The following four models were fitted, using the following sets of features:
-
1.
‘Stage’: overall TNM stage.
-
2.
‘Clinical’: age, clinical T-stage and N-stage, as determined at tumour board review
-
3.
‘ClinVol’: age, clinical T-stage and N-stage, primary CT tumour volume
-
4.
‘ClinRad’: age, clinical T-stage and N-stage, primary tumour volume, two GLCM features.
Discrimination and calibration of 3-year OS were assessed using the area under the receiver operator curve (AUC) and Brier score, respectively, using the riskRegression R library [25]. Confidence intervals were estimated using the method of Blanche [26] and compared using the Delong test. Following Van Rossum [27], clinical utility was assessed by thresholding the model prediction in training data to maximise specificity whilst maintaining sensitivity of > 90%. Confidence intervals for sensitivity, specificity and accuracy were estimated with 1000 replacement bootstraps.
To ensure absence of dataset-partitioning bias or institutional confounding, supplementary post hoc model evaluation was performed using each institution in turn for testing and the remaining four institutions for model fitting. Kaplan–Meier curves were plotted, grouping participants according to the target 90% sensitivity threshold fitted in training data.
A post hoc analysis was also performed to estimate conditional dependencies between radiomic features and survival time, using data from training and testing cohorts. A partial Spearman correlation matrix was inferred using the de-sparsified graphical least absolute shrinkage and selection operator method via the SILGGM package and 95% confidence intervals were estimated via bootstrapping with 1000 replicates. Spearman correlation was also employed to assess volume confounding of radiomic features. Analysis of variance was employed to test radiomic stability with respect to scanner manufacturer and study institution.
Results
Participant and dataset characteristics
Of 210 participants recruited, 48 participants were excluded, generating a training set of 95 participants and a test set of 67 participants. The participant flowchart is provided in Fig. 1 showing the reasons for exclusions. Participant and dataset characteristics are shown in Table 1 and Supplementary Fig. 3.
Performance of model variables
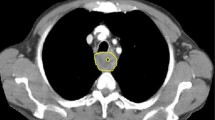
Spearman correlations of the prespecified predictors in the training data are shown in Fig. 2A. GLCM_InverseDifferenceMoment was highly correlated with tumour volume (r = + 0.33) and GLCM_Contrast (r = − 0.94). Hence, GLCM_Correlation and GLCM_Contrast were selected for modelling (Supplementary Material). Clinical T-stage was the most important prognosticator, with each model assigning it a significant positive coefficient (representing increasing risk with increasing stage). The second most influential predictor was age, which was assigned significant positive coefficients in each model. Tumour volume was an insignificant predictor in both the ClinVol and ClinRad models. GLCM_Correlation was assigned a marginal negative coefficient in the ClinRad model, and GLCM_Contrast was the least influential model predictor. Model coefficients, their standard errors and associated z-tests are reported in Table 2. Histograms of model predictions demonstrated that each model had similar distributions of predictions in training and testing (Fig. 2B). Radiomic features are visualised at voxel level in Fig. 3.
Visualisation of CT images and voxel-level radiomic features (GLCM correlation and GLCM contrast) in four study participants. Participant A: 71-year-old female with a clinically staged T2 tumour [correlation 0.67, contrast 1.22]; participant B: 78-year-old female with a clinically staged T3 tumour [correlation 0.5, contrast 2.56]; participant C: 62-year-old male with a clinically staged T2 tumour [correlation 0.48, contrast 3.01]; participant D: 56-year-old male with a clinically staged T3 tumour [correlation 0.57, contrast 2.10]
Prediction of 3-year overall survival
The ClinRad model showed best discrimination of 3-year OS, achieving similar performance in both training (AUC, 0.71 [95% CI 0.60, 0.82]) and testing (AUC, 0.68 [95% CI 0.54, 0.81]) (Table 3). Test discrimination of the ClinRad model was greater than that of Stage alone (Δ AUC, 0.12 [95% CI 0.01, 0.23]; p = 0.04). Stage was the least discriminative model in both training (AUC, 0.60 [95% CI 0.49, 0.71]) and testing (AUC, 0.56 [95% CI 0.44, 0.67]).
The Clinical model achieved similar test discrimination to the ClinRad model (AUC, 0.66 [95% CI 0.51, 0.80]; Δ AUC, 0.02 [95% CI − 0.04, 0.08]; p > 0.05). The ClinVol model attained marginally lower test discrimination than the Clinical model (AUC, 0.65 [95% CI 0.50, 0.79]). All models yielded similar test calibration. Only Stage and ClinRad models achieved target 90% sensitivity in both training and testing. Here, the ClinRad model yielded slightly higher specificity (specificity, 0.19 [95% CI 0.06, 0.33]) than Stage (specificity, 0.14 [95% CI 0.03, 0.26]) at this threshold.
Supplementary per-institution model testing results were consistent with the main external validation results (Supplementary Table 2). Kaplan–Meier curves are provided in Fig. 4. Survival statistics are provided in Supplementary Table 3. In the test data, risk groups assigned by the ClinRad model separated survival curves for the initial 3 years, and convergence was observed at 5 years. However, few participants were assigned to the high-risk group (9/67, 13%). Risk groupings assigned by Clinical and ClinVol models achieved little separation of survival curves in testing. Post hoc partial correlation analysis confirmed that, over both training and validation datasets, the most informative predictors of overall survival time were clinical N-stage (partial ρ, −0.15 [95% CI − 0.33, 0.02]) and age (partial ρ, − 0.14 [95% CI − 0.3, 0.02]). The level of independent predictive information contributed by GLCM_Correlation (partial ρ, 0.10 [95% CI − 0.05, 0.25]) was comparable to that of clinical T-stage (partial ρ, − 0.12 [95% CI − 0.26, 0.04]). Partial correlation analysis results are provided in Supplementary Table 4. Feature variability with respect to scanner manufacturer and study institution are also presented in Supplementary Material.
Discussion
The ability to provide individualised risk–benefit analysis would help to optimise management decisions in potentially resectable oesophageal cancer. Improving prognostication is a step in this direction as prognosis influences treatment decisions made by doctors and patients. Surgery may have an impact on quality of life for up to 12 months post treatment [28] and patients with a poorer prognosis may not fully benefit from a multimodal approach. Initial imaging studies have suggested that radiomic features may have additive prognostic value [11, 12, 18, 21]. However, prespecified models have to demonstrate reliable prediction in external datasets without local refitting. Accordingly, studies need to transition to the evaluation of previously proposed predictors and models, rather than continuing to fit new models with many degrees of freedom to new clinical data [18].
In this prospective multicentre study, we have demonstrated that a multivariate clinicoradiomic prognostic model (ClinRad) incorporating previously identified CT features improved discrimination of 3-year OS compared to TNM staging with an AUC of 0.68 in the test dataset, but offered similar calibration. The Clinical model had similar performance as the ClinRad model with an AUC of 0.66. Both Clinical and ClinRad models retained discriminative capacity between training and testing, though calibration deteriorated, suggesting a distributional mean shift between institutions.
Our findings are concordant with previously published data of Larue et al [12], where the high-dimensional random forest radiomic model with other features achieved AUCs of 0.69 and 0.61 in training and testing, respectively. The direction of radiomic coefficients fitted in this study is consistent with previously published results by Zhang et al [21], who observed increasing GLCM_Correlation in patients with oesophageal adenocarcinoma who responded to chemoradiotherapy. In our model, low GLCM_Correlation was an adverse prognosticator. Our finding that GLCM correlation was the most informative predictor also concurs with Klaasen et al [22]. However, as Klaasen’s model employed a random forest architecture, the directional concordance of results could not be verified.
Zhang et al [21] also observed decreasing GLCM contrast in chemoradiotherapy responders. In our study, GLCM contrast did not affect model predictions substantially, indicating that any prognostic information it encoded was already provided by the other clinical and image-based predictors already modelled.
An advantage of our study is that it incorporated multicentre prospective data, thereby providing realistic conditions for the estimation of model informativeness and generalisability. The imaging equipment and protocols were representative of the varying conditions encountered in clinical practice. The imaging acquisition parameters in this dataset reflected typical clinical practice and variations between institutions, which a radiomic model must be able to accommodate. We noted that GLCM correlation and GLCM contrast varied according to institution and scanner manufacturer respectively. This variability introduces noise which can complicate modelling of the underlying prognostic signal. Clinical deployment of radiomic models requires either that this noise is accommodated or that clinical imaging protocols adapt to acquire images under more standard conditions.
Model validation was performed in test data from three institutions which were unobserved during model development, yielding a realistic estimate of model generalisability in our healthcare system. However, our study had limitations. First, manual segmentation especially of early-stage cancers is subject to intra-reader and inter-reader variability [29]. Second, radiomic approaches are not typically well suited for the identification of new imaging biomarkers, due to the low ratio of events to evaluated variables [18, 20]. It is noteworthy that the ClinRad model fitted here is simpler than that of Larue, whilst matching its training performance, and marginally improving upon its generalisation [12]. However, a necessary cost of this study design is that the other informative radiomic features may have been omitted. Third, although both the ClinRad model and TNM staging demonstrated 90% sensitivity, the low specificity achieved at this threshold is a limitation. Fourth, the improvement in performance between the Clinical and ClinRad model is small and unlikely to change clinical management substantially. Finally, the logistic regression models employed in this analysis were insensitive to nonlinear and nonmonotonic effects.
In conclusion, we have confirmed in a prospective multicentre dataset that previously proposed GLCM features—correlation and contrast—contain incremental prognostic information. The clinicoradiomic model incorporating GLCM correlation and contrast with tumour and nodal stage, age and volume outperformed TNM stage alone in the discrimination of 3-year overall survival. Nevertheless, the level of discrimination remained modest and it is questioned if this will impact on management substantially.
Abbreviations
- AUC:
-
Area under receiver operating characteristic curve
- GLCM:
-
Grey-level co-occurrence matrix
- OS:
-
Overall survival
- TNM:
-
Tumour-node-metastasis
References
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I (2020) Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69:1564–1571
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359:1727–1733
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084
Morgan E, Soerjomataram I, Gavin AT et al (2021) International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut 70:234–242
Sudo K, Xiao L, Wadhwa R et al (2014) Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol 32:3400–3405
Obermannová R, Alsina M, Cervantes A et al (2022) Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:992–1004
Wolfson P, Ho KMA, Bassett P et al (2021) Accuracy of clinical staging for T2N0 oesophageal cancer: systematic review and meta-analysis. Dis Esophagus 34:1–12
Tariman JD, Doorenbos A, Schepp KG, Singhal S, Berry DL (2014) Information needs priorities in patients diagnosed with cancer: a systematic review. J Adv Pract Oncol 2014:115–122
Foley KG, Hills RK, Berthon B et al (2018) Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol 28:428–436
Piazzese C, Foley K, Whybra P, Hurt C, Crosby T, Spezi E (2019) Discovery of stable and prognostic CT-based radiomic features independent of contrast administration and dimensionality in oesophageal cancer. PLoS One 14:e0225550
Larue R, Klaassen R, Jochems A et al (2018) Pre-treatment CT radiomics to predict 3-year overall survival following chemoradiotherapy of esophageal cancer. Acta Oncol 57:1475–1481
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The Image Biomarker Standardization Initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295:328–338
Mason DL (2011) Pydicom: an open source DICOM library. Med Phys 38, 6(10):3493
Umeoka S, Koyama T, Togashi K et al (2006) Esophageal cancer: evaluation with triple-phase dynamic CT–initial experience. Radiology 239:777–783
Umeoka S, Koyama T, Watanabe G et al (2010) Preoperative local staging of esophageal carcinoma using dual-phase contrast-enhanced imaging with multi-detector row computed tomography: value of the arterial phase images. J Comput Assist Tomogr 34:406–412
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
O’Shea RJ, Rookyard C, Withey S, Cook GJR, Tsoka S, Goh V (2022) Radiomic assessment of oesophageal adenocarcinoma: a critical review of 18F-FDG PET/CT. PET/MRI and CT Insights Imaging 13:104
Moons KG, Altman DG, Reitsma JB et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162:W1-73
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Zhang YH, Herlin G, Rouvelas I, Nilsson M, Lundell L, Brismar TB (2018) Texture analysis of computed tomography data using morphologic and metabolic delineation of esophageal cancer-relation to tumor type and neoadjuvant therapy response. Dis Esophagus 32:1–10
Klaassen R, Larue R, Mearadji B et al (2018) Feasibility of CT radiomics to predict treatment response of individual liver metastases in esophagogastric cancer patients. PLoS One 13:e0207362
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379
Shapiro J, van Lanschot JJB, Hulshof M et al (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16:1090–1098
Gerds AT, Ozenne B (2020) riskRegression: risk regression models and prediction scores for survival analysis with competing risks. R package version 5
Blanche P, Dartigues JF, Jacqmin-Gadda H (2013) Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 32:5381–5397
van Rossum PS, Fried DV, Zhang L et al (2016) The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med 57:691–700
Lagergren P, Avery KN, Hughes R et al (2007) Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer 110:686–693
Li F, Li Y, Wang X et al (2022) Inter-observer and intra-observer variability in gross tumor volume delineation of primary esophageal carcinomas based on different combinations of diagnostic multimodal images. Front Oncol 12:817413
Acknowledgements
This manuscript was submitted on behalf of the OCCAMS Consortium.
Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium:
Rebecca C. Fitzgerald1, Paul A.W. Edwards1,2, Nicola Grehan1,5, Barbara Nutzinger1, Aisling M Redmond1, Sujath Abbas1, Adam Freeman1Elizabeth C. Smyth5, Maria O’Donovan1,3, Ahmad Miremadi1,3, Shalini Malhotra1,3, Monika Tripathi1,3, Calvin Cheah1, Hannah Coles1, Matthew Eldridge2, Maria Secrier2, Ginny Devonshire2, Sriganesh Jammula2, Jim Davies4, Charles Crichton4, Nick Carroll5, Richard H.Hardwick5, Peter Safranek5, Andrew Hindmarsh5, Vijayendran Sujendran5, Stephen J. Hayes6,13, Yeng Ang6,7,26, Andrew Sharrocks26, Shaun R. Preston8, Izhar Bagwan8, Vicki Save9, J. Robert O’Neill5,9,20, Olga Tucker10,29, Andrew Beggs10,25, Philippe Taniere10, Sonia Puig10, Gianmarco Contino10, Timothy J. Underwood11,12, Ben L. Grace11, Jesper Lagergren14,22, Andrew Davies14,21, Fuju Chang14,21, Ula Mahadeva14, Francesca D. Ciccarelli21, Grant Sanders15, David Chan15, Ed Cheong16, Bhaskar Kumar16, Loveena Sreedharan16, Irshad Soomro17, Philip Kaye17, John Saunders6,17, Laurence Lovat18, Rehan Haidry18, Michael Scott19, Sharmila Sothi23, George B. Hanna27, Christopher J. Peters27, Krishna Moorthy27, Anna Grabowska28, Richard Turkington30, Damian McManus30, Helen Coleman30, Russell D Petty31, Freddie Bartlett32, Tom D.L. Crosby33
1Early Cancer Institute, Department of Oncology, Hutchison Research Centre, University of Cambridge, Cambridge, CB2 0XZ, UK
2Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, CB2 0RE, UK
3Department of Histopathology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, CB2 0QQ, UK
4Department of Computer Science, University of Oxford, Oxford, OX1 3QD, UK
5Cambridge University Hospitals NHS Foundation Trust, Cambridge, CB2 0QQ, UK
6Salford Royal NHS Foundation Trust, Salford, M6 8HD, UK
7Wigan and Leigh NHS Foundation Trust, Wigan, Manchester, WN1 2NN, UK
8Royal Surrey County Hospital NHS Foundation Trust, Guildford, GU2 7XX, UK
9Edinburgh Royal Infirmary, Edinburgh, EH16 4SA, UK
10University Hospitals Birmingham NHS Foundation Trust, Birmingham, B15 2GW, UK
11University Hospital Southampton NHS Foundation Trust, Southampton, SO16 6YD, UK
12Cancer Sciences Division, University of Southampton, Southampton, SO17 1BJ, UK
13Faculty of Medical and Human Sciences, University of Manchester, Manchester, M13 9PL, UK
14Guy’s and St Thomas’s NHS Foundation Trust, London, SE1 7EH, UK
15Plymouth Hospitals NHS Trust, Plymouth, PL6 8DH, UK
16Norfolk and Norwich University Hospital NHS Foundation Trust, Norwich, NR4 7UY, UK
17Nottingham University Hospitals NHS Trust, Nottingham, NG7 2UH, UK
18University College London, London, WC1E 6BT, UK
19Wythenshawe Hospital, Manchester, M23 9LT, UK
20Edinburgh University, Edinburgh, EH8 9YL, UK
21King’s College London, London, WC2R 2LS, UK
22Karolinska Institute, Stockholm, SE-171 77, Sweden
23University Hospitals Coventry and Warwickshire NHS, Trust, Coventry, CV2 2DX, UK
24Peterborough Hospitals NHS Trust, Peterborough City Hospital, Peterborough, PE3 9GZ, UK
25Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, B15 2TT, UK
26Gastrointestinal Science Centre, University of Manchester, M13 9PL, UK
27Department of Surgery and Cancer, Imperial College, London, W2 1NY, UK
28Queen’s Medical Centre, University of Nottingham, Nottingham, NG7 2UH, UK
29Heart of England NHS Foundation Trust, Birmingham, B9 5SS, UK
30Centre for Cancer Research and Cell Biology, Queen’s University Belfast, Belfast, BT7 1NN, Northern Ireland
31Tayside Cancer Centre, Ninewells Hospital and Medical School, Dundee, DD1 9SY, Scotland, UK
32Portsmouth Hospitals NHS Trust, Portsmouth, PO6 3LY, UK
33Velindre University NHS Trust, Cardiff, Wales, CF15 7QZ, UK
Funding
The authors acknowledge funding support from the following: UK Research & Innovation London Medical Imaging and Artificial Intelligence Centre; Wellcome/Engineering and Physical Sciences Research Council Centre for Medical Engineering at King’s College London [WT 203148/Z/16/Z]; National Institute for Health Research Biomedical Research Centre at Guy’s & St Thomas’ Hospitals and King’s College London; National Institute for Health Research Biomedical Research Centre at Guy’s & St Thomas’ Hospitals and King’s College London; Cancer Research UK National Cancer Imaging Translational Accelerator [C1519/A28682]. OCCAMS was funded by a Programme Grant from Cancer Research UK (RG66287, A15874). OCCAMS2 was funded by a Programme Grant from Cancer Research UK (RG81771/RG84119, A22720/A22131).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Vicky Goh.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
None of these study subjects or cohorts has been previously reported.
Methodology
• prospective
• diagnostic or prognostic study
• multicentre study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Shea, R., Withey, S.J., Owczarczyk, K. et al. Multicentre validation of CT grey-level co-occurrence matrix features for overall survival in primary oesophageal adenocarcinoma. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10666-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10666-y