Abstract
Objectives
To develop a deep-learning method for whole-body fetal segmentation based on MRI; to assess the method’s repeatability, reproducibility, and accuracy; to create an MRI-based normal fetal weight growth chart; and to assess the sensitivity to detect fetuses with growth restriction (FGR).
Methods
Retrospective data of 348 fetuses with gestational age (GA) of 19–39 weeks were included: 249 normal appropriate for GA (AGA), 19 FGR, and 80 Other (having various imaging abnormalities). A fetal whole-body segmentation model with a quality estimation module was developed and evaluated in 169 cases. The method was evaluated for its repeatability (repeated scans within the same scanner, n = 22), reproducibility (different scanners, n = 6), and accuracy (compared with birth weight, n = 7). A normal MRI-based growth chart was derived.
Results
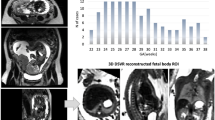
The method achieved a Dice = 0.973, absolute volume difference ratio (VDR) = 1.8% and VDR mean difference = 0.75% (\({CI}_{95\%}\): − 3.95%, 5.46), and high agreement with the gold standard. The method achieved a repeatability coefficient = 4.01%, ICC = 0.99, high reproducibility with a mean difference = 2.21% (\({CI}_{95\%}\): − 1.92%, 6.35%), and high accuracy with a mean difference between estimated fetal weight (EFW) and birth weight of − 0.39% (\({CI}_{95\%}\): − 8.23%, 7.45%). A normal growth chart (n = 246) was consistent with four ultrasound charts. EFW based on MRI correctly predicted birth-weight percentiles for all 18 fetuses ≤ 10thpercentile and for 14 out of 17 FGR fetuses below the 3rd percentile. Six fetuses referred to MRI as AGA were found to be < 3rd percentile.
Conclusions
The proposed method for automatic MRI-based EFW demonstrated high performance and sensitivity to identify FGR fetuses.
Clinical relevance statement
Results from this study support the use of the automatic fetal weight estimation method based on MRI for the assessment of fetal development and to detect fetuses at risk for growth restriction.
Key Points
• An AI-based segmentation method with a quality assessment module for fetal weight estimation based on MRI was developed, achieving high repeatability, reproducibility, and accuracy.
• An MRI-based fetal weight growth chart constructed from a large cohort of normal and appropriate gestational-age fetuses is proposed.
• The method showed a high sensitivity for the diagnosis of small fetuses suspected of growth restriction.





Similar content being viewed by others
Abbreviations
- AGA:
-
Appropriate for gestational age
- aVDR :
-
Absolute volume difference ratio
- EFW:
-
Estimated fetal weight
- FGR:
-
Fetal growth restriction
- FIESTA:
-
Fast imaging employing steady-state acquisition
- GA:
-
Gestational age
- HASTE:
-
Half-Fourier acquisition single-shot turbo spin-echo
- ICC:
-
Intraclass correlation coefficient
- TRUFI:
-
True fast imaging with steady-state free precession
- TTA:
-
Test time augmentations
- VDR :
-
Volume difference ratio
References
Zhang-Rutledge K, Mack LM, Mastrobattista JM, Gandhi M (2018) Significance and outcomes of fetal growth restriction below the 5th percentile compared to the 5th to 10th percentiles on midgestation growth ultrasonography. J Ultrasound Med 37(9):2243–2249. https://doi.org/10.1002/jum.14577
Rizzo G, Mappa I, Bitsadze V et al (2020) Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: prospective cohort study. Ultrasound Obstet Gynecol 55(6):793–798. https://doi.org/10.1002/uog.20406
Peleg D, Kennedy CM, Hunter SK (1998) Intrauterine growth restriction: identification and management. Am Fam Physician 58(2):453
Milner J, Jane A (2018) The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: a systematic review. Ultrasound 26(1):32–41. https://doi.org/10.1177/1742271X17732807
Kacem Y, Cannie MM, Kadji C et al (2013) Fetal weight estimation: comparison of two-dimensional US and MR imaging assessments. Radiology 267(3):902–910. https://doi.org/10.1148/radiol.12121374
Sánchez-Fernández M, Corral ME, Aceituno L, Mazheika M, Mendoza N, Mozas-Moreno J (2021) Observer influence with other variables on the accuracy of Ultrasound estimation of fetal weight at term. Medicina (Kaunas) 57(3):216. https://doi.org/10.3390/medicina57030216
Davidson JR, Uus A, Matthew J et al (2021) Fetal body MRI and its application to fetal and neonatal treatment: an illustrative review. Lancet Child Adolesc Health 5(6):447–458. https://doi.org/10.1016/S2352-4642(20)30313-8
Gholipour A, Estroff JA, Barnewolt CE et al (2014) Fetal MRI: a technical update with educational aspirations. Concepts Magn Reson Part A Bridg Educ Res 43(6):237–266. https://doi.org/10.1002/cmr.a.21321
Uotila J, Dastidar P, Heinonen T, Ryymin P, Punnonen R, Laasonen E (2000) Magnetic resonance imaging compared to ultrasonography in fetal weight and volume estimation in diabetic and normal pregnancy. Acta Obstet Gynecol Scand 79(4):255–259. https://doi.org/10.1034/j.1600-0412.2000.079004255.x
Zaretsky MV, Reichel TF, McIntire DD, Twickler DM (2003) Comparison of magnetic resonance imaging to ultrasound in the estimation of birth weight at term. Am J Obstet Gynecol 189(4):1017–1020. https://doi.org/10.1067/S0002-9378(03)00895-0
Kadji C, Cannie MM, Resta S et al (2019) Magnetic resonance imaging for prenatal estimation of birthweight in pregnancy: review of available data, techniques, and future perspectives. Am J Obstet Gynecol 220(5):428–439. https://doi.org/10.1016/j.ajog.2018.12.031
Torrents-Barrena J, Piella G, Masoller N et al (2019) Segmentation and classification in MRI and US fetal imaging: recent trends and future prospects. Med Image Anal 1(51):61–88. https://doi.org/10.1016/j.media.2018.10.003
Anquez J, Bibin L, Angelini ED, Bloch I (2010) Segmentation of the fetal envelope on ante-natal MRI. Proc IEEE Int Symposium on Biomedical Imaging, pp 896–899. https://doi.org/10.1109/ISBI.2010.5490131
Zhang T, Matthew J, Lohezic M et al (2016) Graph-based whole body segmentation in fetal MR images. Proc MICCAI Work PIPPI, Athens, Greece 21:21
Dudovitch G, Link-Sourani D, Ben Sira L, Miller E, Ben Bashat D, Joskowicz L (2020) Deep learning automatic fetal structures segmentation in MRI scans with few annotated datasets. In Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Cham, pp. 365–374. https://doi.org/10.1007/978-3-030-59725-2_35
Lo J, Nithiyanantham S, Cardinell J et al (2021) Cross Attention Squeeze Excitation Network (CASE-Net) for whole body fetal MRI segmentation. Sensors (Basel) 21(13):4490. https://doi.org/10.3390/s21134490
Ryd D, Nilsson A, Heiberg E, Hedstrom E (2022) Automatic segmentation of the fetus in 3D magnetic resonance images using deep learning: accurate and fast fetal volume quantification for clinical use. Pediatr Cardiol. https://doi.org/10.1007/s00246-022-03038-0
Bartlett JW, Frost C (2008) Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 31(4):466–475. https://doi.org/10.1002/uog.5256
Specktor-Fadida B, Link-Sourani D, Ferster-Kveller S et al (2021) A bootstrap self-training method for sequence transfer: state-of-the-art placenta segmentation in fetal MRI. In Proc. Uncertainty for Safe Utilization of Machine Learning in Medical Imaging, and Perinatal Imaging, Placental and Preterm Image Analysis. Springer, Cham, pp 189–199. https://doi.org/10.1007/978-3-030-87735-4_18
Isensee F, Jaeger PF, Kohl SA, Petersen J, Maier-Hein KH (2021) nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 18(2):203–211. https://doi.org/10.1038/s41592-020-01008-z
Wang G, Li W, Aertsen M, Deprest J, Ourselin S, Vercauteren T (2019) Aleatoric uncertainty estimation with test-time augmentation for medical image segmentation with convolutional neural networks. Neurocomputing 21(338):34–45. https://doi.org/10.1016/j.neucom.2019.01.103
Daniel-Spiegel E, Weiner E, Yarom I et al (2013) Establishment of fetal biometric charts using quantile regression analysis. J Ultrasound Med 32(1):23–33. https://doi.org/10.7863/jum.2013.32.1.23
Hadlock FP, Harrist RB, Martinez-Poyer J (1991) In utero analysis of fetal growth: a sonographic weight standard. Radiology 181(1):129–133. https://doi.org/10.1148/radiology.181.1.1887021
Kiserud T, Piaggio G, Carroli G et al (2017) The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med 14(1):e1002220. https://doi.org/10.1371/journal.pmed.1002220
Stirnemann J, Villar J, Salomon LJ et al (2017) International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol 49(4):478–486. https://doi.org/10.1002/uog.17347
Nicolaides KH, Wright D, Syngelaki A, Wright A, Akolekar R (2018) Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol 52(1):44–51. https://doi.org/10.1002/uog.19073
Dudley NJ (2005) A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol 25(1):80–89. https://doi.org/10.1002/uog.1751
Raunig DL, McShane LM, Pennello G et al (2015) Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res 24(1):27–67. https://doi.org/10.1177/0962280214537344
Joskowicz L, Cohen D, Caplan N, Sosna J (2019) Inter-observer variability of manual contour delineation of structures in CT. Eur Radiol 29(3):1391–1399. https://doi.org/10.1007/s00330-018-5695-5
Liao K, Tang L, Peng C et al (2019) A modified model can improve the accuracy of fetal weight estimation by magnetic resonance imaging. Eur J Radiol 1(110):242–248. https://doi.org/10.1016/j.ejrad.2018.12.009
Schild RL (2007) Three-dimensional volumetry and fetal weight measurement. Ultrasound Obstet Gynecol 30(6):799–803. https://doi.org/10.1002/uog.5181
Baker PN, Johnson IR, Gowland PA et al (1994) Fetal weight estimation by echo-planar magnetic resonance imaging. Lancet 343(8898):644–645. https://doi.org/10.1016/S0140-6736(94)92638-7
Shinozuka N, Okai T, Kohzuma S et al (1987) Formulas for fetal weight estimation by ultrasound measurements based on neonatal specific gravities and volumes. Am J Obstet Gynecol 157(5):1140–1145. https://doi.org/10.1016/S0002-9378(87)80278-8
McCrindle B, Zukotynski K, Doyle TE, Noseworthy MD (2021) A radiology-focused review of predictive uncertainty for AI interpretability in computer-assisted segmentation. Radiol Artif Intell 3(6). https://doi.org/10.1148/ryai.2021210031
Acknowledgements
This research was supported by Kamin grants 63418 and 72126 from the Israel Innovation Authority. We are grateful to the MRI technicians for scanning the fetuses, to the mothers who participated, and to those involved in data preparation and annotation: Ori Benzvi helped with data extraction, and Tuvia Ganot, Kerina Krupnik, Cassandra Kapoor, and Shelley Levi helped with the annotation process and data transfer.
Funding
This work was financially supported by Kamin grants of the Israel Innovation Authority.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dafna Ben-Bashat.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Boards of Tel Aviv Sourasky Medical Center and Children’s Hospital of Eastern Ontario for the retrospective data and was obtained from all participants in the prospective data at Tel Aviv Sourasky Medical Center.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Study subjects have not been previously reported.
Methodology
• prospective and retrospective
• cross-sectional study
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Specktor-Fadida, B., Link-Sourani, D., Rabinowich, A. et al. Deep learning–based segmentation of whole-body fetal MRI and fetal weight estimation: assessing performance, repeatability, and reproducibility. Eur Radiol 34, 2072–2083 (2024). https://doi.org/10.1007/s00330-023-10038-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10038-y