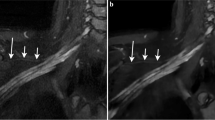
Abstract
Objectives
To explore the use of deep learning–constrained compressed sensing (DLCS) in improving image quality and acquisition time for 3D MRI of the brachial plexus.
Methods
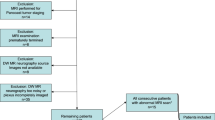
Fifty-four participants who underwent contrast-enhanced imaging and forty-one participants who underwent unenhanced imaging were included. Sensitivity encoding with an acceleration of 2 × 2 (SENSE4x), CS with an acceleration of 4 (CS4x), and DLCS with acceleration of 4 (DLCS4x) and 8 (DLCS8x) were used for MRI of the brachial plexus. Apparent signal-to-noise ratios (aSNRs), apparent contrast-to-noise ratios (aCNRs), and qualitative scores on a 4-point scale were evaluated and compared by ANOVA and the Friedman test. Interobserver agreement was evaluated by calculating the intraclass correlation coefficients.
Results
DLCS4x achieved higher aSNR and aCNR than SENSE4x, CS4x, and DLCS8x (all p < 0.05). For the root segment of the brachial plexus, no statistically significant differences in the qualitative scores were found among the four sequences. For the trunk segment, DLCS4x had higher scores than SENSE4x (p = 0.04) in the contrast-enhanced group and had higher scores than SENSE4x and DLCS8x in the unenhanced group (all p < 0.05). For the divisions, cords, and branches, DLCS4x had higher scores than SENSE4x, CS4x, and DLCS8x (all p ≤ 0.01). No overt difference was found among SENSE4x, CS4x, and DLCS8x in any segment of the brachial plexus (all p > 0.05).
Conclusions
In three-dimensional MRI for the brachial plexus, DLCS4x can improve image quality compared with SENSE4x and CS4x, and DLCS8x can maintain the image quality compared to SENSE4x and CS4x.
Clinical relevance statement
Deep learning–constrained compressed sensing can improve the image quality or accelerate acquisition of 3D MRI of the brachial plexus, which should be benefit in evaluating the brachial plexus and its branches in clinical practice.
Key Points
•Deep learning–constrained compressed sensing showed higher aSNR, aCNR, and qualitative scores for the brachial plexus than SENSE and CS at the same acceleration factor with similar scanning time.
•Deep learning–constrained compressed sensing at acceleration factor of 8 had comparable aSNR, aCNR, and qualitative scores to SENSE4x and CS4x with approximately half the examination time.
•Deep learning–constrained compressed sensing may be helpful in clinical practice for improving image quality and acquisition time in three-dimensional MRI of the brachial plexus.




Similar content being viewed by others
Abbreviations
- aCNR:
-
Apparent contrast-to-noise ratio
- aSNR:
-
Apparent signal-to-noise ratio
- CS:
-
Compressed sensing
- CS4x:
-
Compressed sensing with an acceleration factor of 4
- DLCS:
-
Deep learning–constrained CS
- DLCS4x:
-
DLCS with an acceleration factor of 4
- DLCS8x:
-
DLCS with an acceleration factor of 8
- MRN:
-
Magnetic resonance neurography
- SENSE4x:
-
Sensitivity encoding with an acceleration factor of 2 × 2
References
Bowen BC, Pattany PM, Saraf-Lavi E, Maravilla KR (2004) The brachial plexus: normal anatomy, pathology, and MR imaging. Neuroimaging Clin N Am 14:59–85
Rehman I, Chokshi FH, Khosa F (2014) MR imaging of the brachial plexus. Clin Neuroradiol 24(3):207–216
Boulter DJ, Job J, Expert Panel on Neurological and Musculoskeletal Imaging et al (2021) ACR Appropriateness Criteria® plexopathy: 2021 update. J Am Coll Radiol 18(11S):S423–S441
van Es HW, Bollen TL, van Heesewijk HPM (2010) MRI of the brachial plexus: a pictorial review. Eur J Radiol 74(2):391–402
Madhuranthakam A, Lenkinski R (2015) Technical advancements in MR neurography. Semin Musculoskelet Radiol 19(2):86–93
Davidson EJ, Tan ET, Pedrick EG, Sneag DB (2023) Brachial plexus magnetic resonance neurography: technical challenges and solutions. Invest Radiol 58(1):14–27
Wang X, Harrison C, Mariappan YK et al (2017) MR neurography of brachial plexus at 3.0 T with robust fat and blood suppression. Radiology 283(2):538–546
Madhuranthakam AJ, Yu H, Shimakawa A et al (2010) T(2)-weighted 3D fast spin echo imaging with water-fat separation in a single acquisition. J Magn Reson Imaging 32(3):745–751
Vargas MI, Gariani J, Delattre BA, Dietemann J-L, Lovblad K, Becker M (2015) Three-dimensional MR imaging of the brachial plexus. Semin Musculoskelet Radio 19(2):137–148
Chhabra A, Thawait GK, Soldatos T et al (2013) High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradio 34(3):486–497
Viallon M, Vargas MI, Jlassi H, Lövblad KO, Delavelle J (2008) High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (short term inversion recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol 18(5):1018–1023
Gilcrease-Garcia BM, Deshmukh SD, Parsons MS (2020) Anatomy, imaging, and pathologic conditions of the brachial plexus. Radiographics 40(6):1686–1714
Queler SC, Tan ET, Geannette C, Prince M, Sneag DB (2021) Ferumoxytol-enhanced vascular suppression in magnetic resonance neurography. Skeletal Radiol 50(11):2255–2266
Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42(5):952–962
Griswold MA, Jakob PM, Heidemann RM et al (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47(6):1202–1210
Donoho DL (2006) Compressed sensing. IEEE Trans Inf Theory 52(4):1289–1306
Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H (2017) Compressed sensing for body MRI. J Magn Reson Imaging 45(4):966–987
Aoike T, Fujima N, Yoneyama M et al (2022) Development of three-dimensional MR neurography using an optimized combination of compressed sensing and parallel imaging. Magn Reson Imaging 87:32–37
Ikeda H, Ohno Y, Murayama K et al (2021) Compressed sensing and parallel imaging accelerated T2 FSE sequence for head and neck MR imaging: comparison of its utility in routine clinical practice. Eur J Radiol 135:109501
Suh CH, Jung SC, Lee HB, Cho SJ (2019) High-resolution magnetic resonance imaging using compressed sensing for intracranial and extracranial arteries: comparison with conventional parallel imaging. Korean J Radiol 20(3):487–497
Cho SJ, Choi YJ, Chung SR, Lee JH, Baek JH (2019) High-resolution MRI using compressed sensing-sensitivity encoding (CS-SENSE) for patients with suspected neurovascular compression syndrome: comparison with the conventional SENSE parallel acquisition technique. Clin Radiol 74(10):817.e9-817.e14
Bustin A, Fuin N, Botnar RM, Prieto C (2020) From compressed-sensing to artificial intelligence-based cardiac MRI reconstruction. Front Cardiovasc Med 7:17
Zhang J, Ghanem B (2018) ISTA-Net: interpretable optimization-inspired deep network for image compressive sensing. 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR). IEEE, pp 1828–1837
Pezzotti N, Yousefi S, Elmahdy MS et al (2020) An adaptive intelligence algorithm for undersampled knee MRI reconstruction. IEEE Access 8:204825–204838
Foreman SC, Neumann J, Han J et al (2022) Deep learning–based acceleration of compressed sense MR imaging of the ankle. Eur Radiol 32(12):8376–8385
Yoshida M, Nakaura T, Inoue T et al (2018) Magnetic resonance cholangiopancreatography with GRASE sequence at 3.0T: does it improve image quality and acquisition time as compared with 3D TSE? Eur Radiol 28(6):2436–2443
Reeder SB, Yu H, Johnson JW et al (2006) T1- and T2-weighted fast spin-echo imaging of the brachial plexus and cervical spine with IDEAL water–fat separation. J Magn Reson Imaging 24(4):825–832
Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M (2010) New approaches in imaging of the brachial plexus. Eur J Radiol 74(2):403–410
Ueda T, Ohno Y, Yamamoto K et al (2021) Compressed sensing and deep learning reconstruction for women’s pelvic MRI denoising: utility for improving image quality and examination time in routine clinical practice. Eur J Radiol 134:109430
Nair PP, Mariappan YK, Paruthikunnan SM et al (2021) Magnetic resonance neurography of the brachial plexus using 3D SHINKEI: comparative evaluation with conventional magnetic resonance sequences for the visualization of anatomy and detection of nerve injury at 1.5T. J Med Phys 46(3):140–147
Cervantes B, Bauer JS, Zibold F et al (2016) Imaging of the lumbar plexus: optimized refocusing flip angle train design for 3D TSE. J Magn Reson Imaging 43(4):789–799
Sneag DB, Daniels SP, Geannette C et al (2020) Post-contrast 3D inversion recovery magnetic resonance neurography for evaluation of branch nerves of the brachial plexus. Eur J Radiol 132:109304
Yarach U, Saekho S, Setsompop K et al (2021) Feasibility of accelerated 3D T1-weighted MRI using compressed sensing: application to quantitative volume measurements of human brain structures. MAGMA 34(6):915–927
Chen Z, Sun B, Xue Y et al (2021) Comparing compressed sensing breath-hold 3D MR cholangiopancreatography with two parallel imaging MRCP strategies in main pancreatic duct and common bile duct. Eur J Radiol 142:109833
Morita K, Nakaura T, Maruyama N et al (2020) Hybrid of compressed sensing and parallel imaging applied to three-dimensional isotropic T2-weighted turbo spin-echo MR imaging of the lumbar spine. Magn Reson Med Sci 19(1):48–55
Sun L, Fan Z, Fu X, Huang Y, Ding X, Paisley J (2019) A deep information sharing network for multi-contrast compressed sensing MRI reconstruction. IEEE Trans Image Process 28(12):6141–6153
Acknowledgements
We thank Hans Peeters (Philips, Best, the Netherlands) for the technical help of this study.
Funding
This study has received funding by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (grant number: ZYGD18019) and Sichuan Province Science and Technology Support Program (grant number: 22QYCX0106).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Zhen-lin Li.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: three authors of this manuscript (Xiao-yong Zhang, Chun-tang Ling, and Hai-Xia Li) are employees of Philips Healthcare, and they are participants in the technique support and analysis of the data. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the manuscript. Zhen-Lin Li of West China Hospital controls the data.
Statistics and biometry
One of the authors (Hai-xia Li) has significant statistical expertise.
Informed consent
Written informed consent was obtained from each subject in this study.
Ethical approval
Institutional Review Board approval was obtained from the Biomedical Research Ethics Committee of West China Hospital.
Study subjects or cohorts overlap
None.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Sx., Xiao, Y., Peng, Wl. et al. Accelerated 3D MR neurography of the brachial plexus using deep learning–constrained compressed sensing. Eur Radiol 34, 842–851 (2024). https://doi.org/10.1007/s00330-023-09996-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09996-0