Abstract
Objectives
To investigate the value of pre-treatment quantitative synthetic MRI (SyMRI) for predicting a good response to neoadjuvant chemoradiotherapy (nCRT) in patients with locally advanced rectal cancer.
Methods
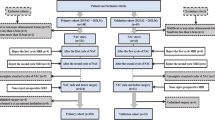
This prospective study enrolled 63 patients with locally advanced rectal cancer scheduled to undergo preoperative chemoradiotherapy from January 2019 to June 2021. T1 relaxation time (T1), T2 relaxation time (T2), proton density (PD) from synthetic MRI, and apparent diffusion coefficient (ADC) from diffusion-weighted imaging (DWI) were measured. Independent-sample t-test, the Mann–Whitney U test, the Delong test, and receiver operating characteristic curve (ROC) analyses were used to predict the pathologic complete response (pCR) and T-downstaging.
Results
Among the 63 patients, 19 (30%) achieved pCR and 44 (70%) did not, and 24 (38%) achieved T-downstaging, while 44 (62%) did not. The mean T1 and T2 values were significantly lower in the pCR group compared with those in the non-pCR group and in the T-downstage group compared with those in the non-T-downstage group (all p < 0.05). There were no significant differences in the PD and ADC values between the two groups. There were no significant differences between the mean values of T1 and T2 for predicting pCR after CRT (AUC, 0.767 vs. 0.831, p = 0.37). There were no significant differences between the AUC values of T1 and T2 values for the assessment of post-CRT T-downstaging (AUC, 0.746 vs. 0.820, p = 0.506).
Conclusions
In patients with locally advanced rectal cancer, the synthetic MRI–derived T1 relaxation time and T2 relaxation time values are promising imaging markers for predicting a good response to neoadjuvant chemoradiotherapy.
Key Points
• Mean T1 and T2 values were significantly lower in the pathologic complete response group and the T-downstage group.
• There were no significant differences in the proton density and apparent diffusion coefficient values between the two groups.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the receiver operating characteristic curve
- DWI:
-
Diffusion-weighted imaging
- LARC:
-
Locally advanced rectal cancer
- MAGIC:
-
Magnetic resonance image compilation
- nCRT:
-
Neoadjuvant chemoradiotherapy
- pCR:
-
Pathologic complete response
- PD:
-
Proton density
- SyMRI:
-
Synthetic MRI
- TRG:
-
Tumor regression grading
References
Gabriel E, Ostapoff K, Attwood K et al (2017) Disparities in the age-related rates of colorectal cancer in the United States. Am Surg 83:640–647
Glynne-Jones R, Wyrwicz L, Tiret E et al (2017) ESMO Guidelines Committee: rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28 (Suppl_4): iv22-iv40
Bigness A, Imanirad I, Sahin IH et al (2021) Locally advanced rectal adenocarcinoma: treatment sequences, intensification, and rectal organ preservation. CA Cancer J Clin 71:198–208
Conroy T, Bosset JF, Etienne PL et al (2021) Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22:702–715
Benson AB, Venook AP, Al-Hawary MM et al (2018) Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:874–901
Habr-Gama A, Perez RO, Nadalin W et al (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240:711–718 discussion 717–718
van der Valk MJM, Hilling DE, Bastiaannet E et al (2018) Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391:2537–2545
Al-Sukhni E, Attwood K, Mattson DM et al (2016) Predictors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Oncol 23:1177–1186
Huang SH, Chi P, Lin HM et al (2016) Selecting stage ypT0-1N0 for locally advanced rectal cancer following preoperative chemoradiotherapy: implications for potential candidates of organ-sparing management. Colorectal Dis 18:989–996
Creavin B, Ryan E, Martin ST et al (2017) Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br J Cancer 116:169–174
Herman JM, Narang AK, Griffith KA et al (2013) The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 85:e15–e19
Duldulao MP, Lee W, Streja L et al (2013) Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 56:142–149
Seo N, Kim H, Cho MS, Lim JS (2019) Response assessment with MRI after chemoradiotherapy in rectal cancer: current evidences. Korean J Radiol 20:1003–1018
Zhang S, Yu M, Chen D et al (2022) Role of MRI based radiomics in locally advanced rectal cancer. Review. Oncol Rep 12:47
Rafaelsen SR, Vagn-Hansen C, Sorensen T et al (2013) Ultrasound elastography in patients with rectal cancer treated with chemoradiation. Eur J Radiol 82:913–917
Warntjes JB, Leinhard OD, West J, Lundberg P (2008) Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med 60:320–329
Hagiwara A, Fujimoto K, Kamagata K et al (2021) Age-related changes in relaxation times, proton density, myelin, and tissue volumes in adult brain analyzed by 2-dimensional quantitative synthetic magnetic resonance imaging. Invest Radiol 56:163–172
Cui Y, Han S, Liu M et al (2020) Diagnosis and grading of prostate cancer by relaxation maps from synthetic MRI. J Magn Reson Imaging 52:552–564
Kumar NM, Fritz B, Stern SE et al (2018) Synthetic MRI of the knee: phantom validation and comparison with conventional MRI. Radiology 289:465–477
Hagiwara A, Warntjes M, Hori M et al (2017) SyMRI of the brain: rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic Brain segmentation, and myelin measurement. Invest Radiol 52:647–657
Roux M, Hilbert T, Hussami M et al (2019) MRI T2 mapping of the knee providing synthetic morphologic images: comparison to conventional turbo Spin-Echo MRI. Radiology 293:620–630
Gao W, Zhang S, Guo J et al (2021) Investigation of synthetic relaxometry and diffusion measures in the differentiation of benign and malignant breast lesions as compared to BI-RADS. J Magn Reson Imaging 53:1118–1127
Chaland B, Mariette F, Marchal P, De Certaines J (2000) 1H nuclear magnetic resonance relaxometric characterization of fat and water states in soft and hard cheese. J Dairy Res 67:609–618
Zaman A, Higgins DM, Motwani M et al (2015) Robust myocardial T2 and T2 * mapping at 3T using image-based shimming. J Magn Reson Imaging 41:1013–1020
Cai Q, Wen Z, Huang Y et al (2021) Investigation of synthetic magnetic resonance imaging applied in the evaluation of the tumor grade of bladder cancer. J Magn Reson Imaging 54:1989–1997
Ge YX, Hu SD, Wang Z et al (2021) Feasibility and reproducibility of T2 mapping and DWI for identifying malignant lymph nodes in rectal cancer. Eur Radiol 31:3347–3354
Zhao L, Liang M, Xie L et al (2021) Prediction of pathological prognostic factors of rectal cancer by relaxation maps from synthetic magnetic resonance imaging. Eur J Radiol 138:109658
Liu L, Yin B, Shek K et al (2018) Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J Int Med Res 46:1928–1935
Boustani J, Grapin M, Laurent PA et al (2019) The 6th R of radiobiology: reactivation of Anti-Tumor Immune Response. Cancers (Basel) 06-20(6):11
Zhang J, Ge Y, Zhang H et al (2022) Quantitative T2 mapping to discriminate mucinous from nonmucinous adenocarcinoma in rectal cancer: comparison with diffusion-weighted imaging. Magn Reson Med Sci 21:593–598
Chand M, Yu S, Swift RI et al (2014) Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol 18:335–344
Fernandes JL, Rochitte CE (2015) T1 mapping: technique and applications. Magn Reson Imaging Clin N Am 23:25–34
Peng WL, Zhang TJ, Shi K et al (2022) Automatic machine learning based on native T1 mapping can identify myocardial fibrosis in patients with hypertrophic cardiomyopathy. Eur Radiol 32:1044–1053
Steinkohl E, Olesen SS, Hansen TM (2021) T1 relaxation times and MR elastography-derived stiffness: new potential imaging biomarkers for the assessment of chronic pancreatitis. Abdom Radiol (NY) 46:5598–5608
Ji S, Yang D, Lee J et al (2022) Synthetic MRI: technologies and applications in neuroradiology. J Magn Reson Imaging 55:1013–1025
Jacobs L, Intven M, van Lelyveld N et al (2016) Diffusion-weighted MRI for early prediction of treatment response on preoperative chemoradiotherapy for patients with locally advanced rectal cancer: a feasibility study. Ann Surg 263:522–528
Xie H, Sun T, Chen M et al (2015) Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 94:e517
Yu J, Xu Q, Song JC et al (2017) The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 27:1848–1857
Hu F, Tang W, Sun Y et al (2017) The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget 8:75597–75606
Acknowledgements
We thank to Qian Long and Zhong Rui for their support.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81902638).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chuanmiao Xie, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Each enrolled patient was asked to sign an informed consent form.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lian, S., Liu, H., Meng, T. et al. Quantitative synthetic MRI for predicting locally advanced rectal cancer response to neoadjuvant chemoradiotherapy. Eur Radiol 33, 1737–1745 (2023). https://doi.org/10.1007/s00330-022-09191-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09191-7