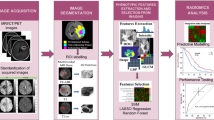
Abstract
Objectives
To test the feasibility of using 3D MRF maps with radiomics analysis and machine learning in the characterization of adult brain intra-axial neoplasms.
Methods
3D MRF acquisition was performed on 78 patients with newly diagnosed brain tumors including 33 glioblastomas (grade IV), 6 grade III gliomas, 12 grade II gliomas, and 27 patients with brain metastases. Regions of enhancing tumor, non-enhancing tumor, and peritumoral edema were segmented and radiomics analysis with gray-level co-occurrence matrices and gray-level run-length matrices was performed. Statistical analysis was performed to identify features capable of differentiating tumors based on type, grade, and isocitrate dehydrogenase (IDH1) status. Receiver operating curve analysis was performed and the area under the curve (AUC) was calculated for tumor classification and grading. For gliomas, Kaplan-Meier analysis for overall survival was performed using MRF T1 features from enhancing tumor region.
Results
Multiple MRF T1 and T2 features from enhancing tumor region were capable of differentiating glioblastomas from brain metastases. Although no differences were identified between grade 2 and grade 3 gliomas, differentiation between grade 2 and grade 4 gliomas as well as between grade 3 and grade 4 gliomas was achieved. MRF radiomics features were also able to differentiate IDH1 mutant from the wild-type gliomas. Radiomics T1 features for enhancing tumor region in gliomas correlated to overall survival (p < 0.05).
Conclusion
Radiomics analysis of 3D MRF maps allows differentiating glioblastomas from metastases and is capable of differentiating glioblastomas from metastases and characterizing gliomas based on grade, IDH1 status, and survival.
Key Points
• 3D MRF data analysis using radiomics offers novel tissue characterization of brain tumors.
• 3D MRF with radiomics offers glioma characterization based on grade, IDH1 status, and overall patient survival.





Similar content being viewed by others
Abbreviations
- ATRX:
-
Alpha thalassemia mental retardation syndrome X-linked
- AUC:
-
Area under the curve
- CE:
-
Contrast enhanced
- ED:
-
Peritumoral edema
- ET:
-
Enhancing tumor
- FLAIR:
-
Fluid-attenuated inversion recovery
- FLIRT:
-
FMRIB Software Library’s Linear Image Registration Tool
- GBs:
-
Glioblastomas
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- ICC:
-
Interobserver concordance
- IDH1:
-
Isocitrate dehydrogenase 1
- IMC1:
-
Information measure of correlation
- IMC2:
-
Information measure of correlation 2
- METs:
-
Metastases
- MRF:
-
Magnetic resonance fingerprinting
- MRI:
-
Magnetic resonance imaging
- NET:
-
Nonenhancing tumor or necrosis
- NSCLC:
-
Non-small cell lung cancer
- RLNN:
-
Run-length nonuniformity normalized
- ROI:
-
Region of interest
- RSF:
-
Random survival forest
- WHO:
-
World Health Organization
References
Wang S, Meng M, Zhang X et al (2018) Texture analysis of diffusion weighted imaging for the evaluation of glioma heterogeneity based on different regions of interest. Oncol Lett 15:7297–7304
Ryu YJ, Choi SH, Park SJ et al (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9:e108335. https://doi.org/10.1371/journal.pone.0108335
O’Connor JPB, Aboagye EO, Adams JE et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186
Haubold J, Demircioglu A, Gratz M et al (2020) Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric 18F-FET PET-MRI and MR fingerprinting. Eur J Nucl Med Mol Imaging 47:1435–1445. https://doi.org/10.1007/s00259-019-04602-2
Ma D, Gulani V, Seiberlich N et al (2013) Magnetic resonance fingerprinting. Nature 495:187–192
Liu Y, Hamilton J, Rajagopalan S, Seiberlich N (2018) Cardiac magnetic resonance fingerprinting: technical overview and initial results. JACC Cardiovascular Imaging 11:1837–1853. https://doi.org/10.1016/j.jcmg.2018.08.028
Wang CY, Coppo S, Mehta BB et al (2019) Magnetic resonance fingerprinting with quadratic RF phase for measurement of T2 * simultaneously with δf , T1 , and T2. Magn Reson Med 81:1849–1862. https://doi.org/10.1002/mrm.27543
Badve C, Yu A, Dastmalchian S et al (2017) MR fingerprinting of adult brain tumors: initial experience. AJNR Am J Neuroradiol 38:492–499
Dastmalchian S, Kilinc O, Onyewadume L et al (2021) Radiomic analysis of magnetic resonance fingerprinting in adult brain tumors. Eur J Nucl Med Mol Imaging 48:683–693. https://doi.org/10.1007/s00259-020-05037-w
Onyewadume L, Kilinc O, Ghodsara S, et al (ISMRM 2018) Radiomic analysis of 3D MR fingerprinting in adult brain tumors. https://archive.ismrm.org/2018/0957.html. Accessed 10 May 2022.
de Blank P, Badve C, Gold DR et al (2019) Magnetic resonance fingerprinting to characterize childhood and young adult brain tumors. Pediatr Neurosurg 54:310–318. https://doi.org/10.1159/000501696
Badve C, Yu A, Rogers M et al (2015) Simultaneous T1 and T2 brain relaxometry in asymptomatic volunteers using magnetic resonance fingerprinting. Tomography:136–144
Chen Y, Chen M-H, Baluyot KR et al (2019) MR fingerprinting enables quantitative measures of brain tissue relaxation times and myelin water fraction in the first five years of life. Neuroimage 186:782–793
Liao C, Wang K, Cao X et al (2018) Detection of lesions in mesial temporal lobe epilepsy by using MR fingerprinting. Radiology 288:804–812
Coristine A, Hamilton J, van Heeswijk RB, et al (2018). Cardiac magnetic resonance fingerprinting in heart transplant recipients. http://archive.ismrm.org/2018/0675.html. Accessed 21 Oct 2021.
Panda A, Obmann VC, Lo W-C et al (2019) MR fingerprinting and ADC mapping for characterization of lesions in the transition zone of the prostate gland. Radiology 292:685–694. https://doi.org/10.1148/radiol.2019181705
Yu AC, Badve C, Ponsky LE et al (2017) Development of a combined MR fingerprinting and diffusion examination for prostate cancer. Radiology 283:729–738. https://doi.org/10.1148/radiol.2017161599
Shiradkar R, Panda A, Leo P et al (2020) T1 and T2 MR fingerprinting measurements of prostate cancer and prostatitis correlate with deep learning-derived estimates of epithelium, lumen, and stromal composition on corresponding whole mount histopathology. Eur Radiol 31:1336–1346
Chen Y, Jiang Y, Pahwa S et al (2016) MR fingerprinting for rapid quantitative abdominal imaging. Radiology 279:278–286
Jaubert O, Cruz G, Bustin A et al (2020) Free-running cardiac magnetic resonance fingerprinting: joint T1/T2 map and cine imaging. Magn Reson Imaging 68:173–182. https://doi.org/10.1016/j.mri.2020.02.005
Cloos MA, Assländer J, Abbas B et al (2019) Rapid radial T1 and T2 mapping of the hip articular cartilage with magnetic resonance fingerprinting. J Magn Reson Imaging 50:810–815. https://doi.org/10.1002/jmri.26615
Ma D, Jones SE, Deshmane A et al (2019) Development of high-resolution 3D MR fingerprinting for detection and characterization of epileptic lesions. J Magn Reson Imaging 49:1333–1346
Hamilton JI, Jiang Y, Chen Y et al (2017) MR fingerprinting for rapid quantification of myocardial T1 , T2 , and proton spin density. Magn Reson Med 77:1446–1458. https://doi.org/10.1002/mrm.26216
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. https://doi.org/10.1016/s1053-8119(02)91132-8
Haralick RM (1979) Statistical and structural approaches to texture. Proceedings of the IEEE 67:786–804
Lupo JM, Cha S, Chang SM, Nelson SJ (2005) Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas: characterization of spatial heterogeneity. AJNR Am J Neuroradiol 26:1446–1454
Hambardzumyan D, Bergers G (2015) Glioblastoma: defining tumor niches. Trends Cancer 1:252–265
Wagner M, Nafe R, Jurcoane A et al (2011) Heterogeneity in malignant gliomas: a magnetic resonance analysis of spatial distribution of metabolite changes and regional blood volume. J Neurooncol 103:663–672. https://doi.org/10.1007/s11060-010-0443-y
Barajas RF, Phillips JJ, Parvataneni R et al (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 14:942–954
Ji B, Wang S, Liu Z et al (2019) Revealing hemodynamic heterogeneity of gliomas based on signal profile features of dynamic susceptibility contrast-enhanced MRI. Neuroimage Clin 23:101864. https://doi.org/10.1016/j.nicl.2019.101864
Deoni SCL (2010) Quantitative relaxometry of the brain. Top Magn Reson Imaging 21:101–113
Deoni SCL, Dean DC, O’Muircheartaigh J et al (2012) Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 63:1038–1053. https://doi.org/10.1016/j.neuroimage.2012.07.037
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Lee EJ, terBrugge K, Mikulis D et al (2011) Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol 196:71–76. https://doi.org/10.2214/AJR.10.4752
Soliman RK, Essa AA, Elhakeem AAS et al (2021) Texture analysis of apparent diffusion coefficient (ADC) map for glioma grading: analysis of whole tumoral and peri-tumoral tissue. Diagn Interv Imaging 102:287–295
Duron L, Balvay D, Vande Perre S et al (2019) Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS One 14:e0213459. https://doi.org/10.1371/journal.pone.0213459
Funding
This study has received funding by National Institutes of Health 1R01BB017219 award (Principal Investigator: Dr. Mark Griswold) and 1R01EB016728 award (Principal Investigators: Drs. Mark Griswold and Vikas Gulani). This project was also supported by the Clinical and Translational Science Collaborative (CTSC) of Cleveland which is funded by the National Institutes of Health (NIH), National Center for Advancing Translational Science (NCATS), Clinical and Translational Science Award (CTSA) grant, UL1TR002548 (Principal Investigator: Dr. Chaitra Badve). AES is supported by NIH CA217956, the Peter D Cristal Chair, the Center of Excellence for Translational Neuro Oncol, the Gerald R. Kaufman Fund for Glioma Research at University Hospitals of Cleveland, the Kimble Family Foundation, and the Ferry Family Foundation at University Hospitals of Cleveland. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Chaitra Badve.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Case Western Reserve University and University Hospitals receive research support from Siemens. Chaitra Badve, Dan Ma, Mark Griswold, and Jeffrey Sunshine have patent applications on MRF and its applications. Charit Tippareddy, Louisa Onyewadume, Andrew E. Sloan, Gi-Ming Wang, Nirav T. Patil, Jill S. Barnholtz-Sloan, and Rasim Boyacıoğlu have nothing to disclose.
Statistics and biometry
Three authors have significant statistical expertise: Dr. Jill Barnholtz-Sloan, Gi-Ming Wang, and Nirav Patil.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
-
prospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 72 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tippareddy, C., Onyewadume, L., Sloan, A.E. et al. Novel 3D magnetic resonance fingerprinting radiomics in adult brain tumors: a feasibility study. Eur Radiol 33, 836–844 (2023). https://doi.org/10.1007/s00330-022-09067-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09067-w