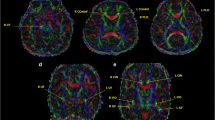
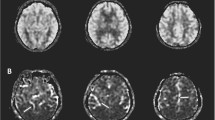
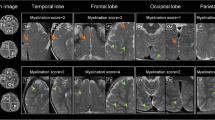
Abstract
Objective
To study longitudinal changes in tuber and whole-brain perfusion in children with tuberous sclerosis complex (TSC) using arterial spin labeling (ASL) perfusion MRI and correlate them with pathological EEG slow wave activity and neurodevelopmental outcomes.
Methods
Retrospective longitudinal cohort study of 13 children with TSC, 3 to 6 serial ASL-MRI scans between 2 months and 7 years of age (53 scans in total), and an EEG examination performed within 2 months of the last MRI. Tuber cerebral blood flow (CBF) values were calculated in tuber segmentation masks, and tuber:cortical CBF ratios were used to study tuber perfusion. Logistic regression analysis was performed to identify which initial tuber characteristics (CBF value, volume, location) in the first MRI predicted tubers subsequently associated with EEG slow waves. Whole-brain and lobar CBF values were extracted for all patient scans and age-matched controls. CBF ratios were compared in patients and controls to study longitudinal changes in whole-brain CBF.
Results
Perfusion was reduced in tubers associated with EEG slow waves compared with other tubers. Low tuber CBF values around 6 months of age and large tuber volumes were predictive of tubers subsequently associated with EEG slow waves. Patients with severe developmental delay had more severe whole-brain hypoperfusion than those with no/mild delay, which became apparent after 2 years of age and were not associated with a higher tuber load.
Conclusions
Dynamic changes in tuber and brain perfusion occur over time. Perfusion is significantly reduced in tubers associated with EEG slow waves. Whole-brain perfusion is significantly reduced in patients with severe delay.
Key Points
• Tubers associated with EEG slow wave activity were significantly more hypoperfused than other tubers, especially after 1 year of age.
• Larger and more hypoperfused tubers at 6 months of age were more likely to subsequently be associated with pathological EEG slow wave activity.
• Patients with severe developmental delay had more extensive and severe global hypoperfusion than those without developmental delay.




Similar content being viewed by others
Abbreviations
- ASD:
-
Autism spectrum disorder
- ASL:
-
Arterial spin labeling
- CBF:
-
Cerebral blood flow
- IQR:
-
Interquartile range
- PET:
-
Positron emission tomography
- ROC:
-
Receiver operating characteristics
- SPECT:
-
Single-photon emission computed tomography
- SPM:
-
Statistical parametric mapping
- TSC:
-
Tuberous sclerosis complex
References
Mühlebner A, van Scheppingen J, Hulshof HM et al (2016) Novel histopathological patterns in cortical tubers of epilepsy surgery patients with tuberous sclerosis complex. PLoS One 11:e0157396–e0157396. https://doi.org/10.1371/journal.pone.0157396
Peters JM, Taquet M, Prohl AK et al (2013) Diffusion tensor imaging and related techniques in tuberous sclerosis complex: review and future directions. Future Neurol 8:583–597. https://doi.org/10.2217/fnl.13.37
Gupta A, de Bruyn G, Tousseyn S et al (2020) Epilepsy and neurodevelopmental comorbidities in tuberous sclerosis complex: a natural history study. Pediatr Neurol 106:10–16. https://doi.org/10.1016/j.pediatrneurol.2019.12.016
Nabbout R, Belousova E, Benedik MP et al (2018) Epilepsy in tuberous sclerosis complex: findings from the TOSCA Study. Epilepsia Open 4:73–84. https://doi.org/10.1002/epi4.12286
Kaczorowska M, Jurkiewicz E, Domańska-Pakieła D et al (2011) Cerebral tuber count and its impact on mental outcome of patients with tuberous sclerosis complex. Epilepsia 52:22–27. https://doi.org/10.1111/j.1528-1167.2010.02892.x
Bolton PF, Park RJ, Higgins JNP et al (2002) Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 125:1247–1255. https://doi.org/10.1093/brain/awf124
Numis AL, Major P, Montenegro MA et al (2011) Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 76:981–987. https://doi.org/10.1212/WNL.0b013e3182104347
Doherty C, Goh S, Poussaint TY et al (2005) Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol 20:837–841. https://doi.org/10.1177/08830738050200101301
Okanishi T, Fujimoto A, Kanai S et al (2020) Association between diffuse cerebral MRI lesions and the occurrence and intractableness of West syndrome in tuberous sclerosis complex. Epilepsy Behav 103:106535. https://doi.org/10.1016/j.yebeh.2019.106535
El-Beheiry A, Nassef H, Darwish R et al (2018) Which cortical tuber type is more epileptogenic? Magnetic resonance imaging-based study in children with tuberous sclerosis complex. Alexandria J Pediatr 31:43. https://doi.org/10.4103/AJOP.AJOP_13_18
Chu-Shore CJ, Major P, Montenegro M, Thiele E (2009) Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology 72:1165–1169. https://doi.org/10.1212/01.wnl.0000345365.92821.86
Jansen FE, Vincken KL, Algra A et al (2008) Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 70:916–923. https://doi.org/10.1212/01.wnl.0000280579.04974.c0
Pollock JM, Whitlow CT, Tan H et al (2009) Pulsed arterial spin-labeled MR imaging evaluation of tuberous sclerosis. AJNR Am J Neuroradiol 30:815–820. https://doi.org/10.3174/ajnr.A1428
Widjaja E, Wilkinson ID, Griffiths PD (2007) Magnetic resonance perfusion imaging in malformations of cortical development. Acta Radiol 48:907–917. https://doi.org/10.1080/02841850701477686
Fedi M, Reutens DC, Andermann F et al (2003) α-[11C]-Methyl-L-tryptophan PET identifies the epileptogenic tuber and correlates with interictal spike frequency. Epilepsy Res 52:203–213. https://doi.org/10.1016/S0920-1211(02)00216-4
Chugani HT, Luat AF, Kumar A et al (2013) α-[11C]-Methyl-L-tryptophan--PET in 191 patients with tuberous sclerosis complex. Neurology 81:674–680. https://doi.org/10.1212/WNL.0b013e3182a08f3f
Koh S, Jayakar P, Resnick T et al (1999) The localizing value of ictal SPECT in children with tuberous sclerosis complex and refractory partial epilepsy. Epileptic Disord 1:41–46
Benova B, Petrak B, Kyncl M et al (2018) Early predictors of clinical and mental outcome in tuberous sclerosis complex: a prospective study. Eur J Paediatr Neurol 22:632–641. https://doi.org/10.1016/j.ejpn.2018.03.001
De Ridder J, Lavanga M, Verhelle B et al (2020) Prediction of neurodevelopment in infants with tuberous sclerosis complex using early EEG characteristics. Front Neurol 11:1151. https://doi.org/10.3389/fneur.2020.582891
Baldini S, Coito A, Korff CM et al (2020) Localizing non-epileptiform abnormal brain function in children using high density EEG: Electric Source Imaging of focal slowing. Epilepsy Res 159:106245. https://doi.org/10.1016/j.eplepsyres.2019.106245
Haller S, Zaharchuk G, Thomas DL et al (2016) Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 281:337–356. https://doi.org/10.1148/radiol.2016150789
Hully M, Grevent D, Breuillard D et al (2016) Arterial spin labeling shows pre-epileptic tuber hyperperfusion in tuberous sclerosis complex. Neurology 86:1744–1745. https://doi.org/10.1212/WNL.0000000000002636
Northrup H, Krueger DA (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:243–254. https://doi.org/10.1016/j.pediatrneurol.2013.08.001
Brunet O, Lézine I, Josse D (2001) Brunet-Lézine révisé : Echelle de développement psychomoteur de la première enfance: manuel BLR-C
Park SE, Demakis GJ (2017) Wechsler preschool and primary scale of intelligence. In: Zeigler-Hill V, Shackelford TK (eds) Encyclopedia of personality and individual differences. Springer International Publishing, Cham, pp 1–4
Sparrow SS, Cicchetti DV, Saulnier CA (2016) Vineland adaptive behavior scales, Third Edition (Vineland-3). NCS Pearson, Bloomington
Rutter M, LeCouteur A, Lord C (2003) The autism diagnostic interview-revised, vol 24. West Psychol Serv, Los Angeles, pp 659–685
Lord C, Rutter M, DiLavore P, Risi S (2008) Autism diagnostic observation schedule (ADOS): manual. Western Psychological Services, Los Angeles
Makeig S, Bell AJ, Jung T-P, Sejnowski TJ (1998) Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst 8:145–151
Tao J, Chen X-J, Baldwin M et al (2011) Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 52:467–476. https://doi.org/10.1111/j.1528-1167.2010.02918.x
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. https://doi.org/10.1006/nimg.2001.0978
Peters JM, Prohl AK, Tomas-Fernandez XK et al (2015) Tubers are neither static nor discrete: evidence from serial diffusion tensor imaging. Neurology 85:1536–1545. https://doi.org/10.1212/WNL.0000000000002055
Nishida M, Asano E, Juhász C et al (2008) Cortical glucose metabolism correlates negatively with delta-slowing and spike-frequency in epilepsy associated with tuberous sclerosis. Hum Brain Mapp 29:1255–1264. https://doi.org/10.1002/hbm.20461
Kielar A, Deschamps T, Chu RKO et al (2016) Identifying dysfunctional cortex: dissociable effects of stroke and aging on resting state dynamics in MEG and fMRI. Front Aging Neurosci 8:40. https://doi.org/10.3389/fnagi.2016.00040
Kulandaivel K, Holmes GL (2011) Power spectral analysis in infants with seizures: relationship to development. Epilepsy Behav 20:700–705. https://doi.org/10.1016/j.yebeh.2011.02.021
Pendse N, Wissmeyer M, Altrichter S et al (2010) Interictal arterial spin-labeling MRI perfusion in intractable epilepsy. J Neuroradiol 37:60–63. https://doi.org/10.1016/j.neurad.2009.05.006
Lemaître H, Augé P, Saitovitch A et al (2021) Rest functional brain maturation during the first year of life. Cereb Cortex 31:1776–1785. https://doi.org/10.1093/cercor/bhaa325
Kotulska K, Kwiatkowski DJ, Curatolo P et al (2021) Prevention of epilepsy in infants with tuberous sclerosis complex in the EPISTOP Trial. Ann Neurol 89:304–314. https://doi.org/10.1002/ana.25956
Samueli S, Dressler A, Gröppel G et al (2018) Everolimus in infants with tuberous sclerosis complex-related West syndrome: first results from a single-center prospective observational study. Epilepsia 59:e142–e146. https://doi.org/10.1111/epi.14529
Fohlen M, Taussig D, Ferrand-Sorbets S et al (2018) Refractory epilepsy in preschool children with tuberous sclerosis complex: early surgical treatment and outcome. Seizure 60:71–79. https://doi.org/10.1016/j.seizure.2018.06.005
Joo EY, Hong S, Tae W-S et al (2006) Effect of lamotrigine on cerebral blood flow in patients with idiopathic generalised epilepsy. Eur J Nucl Med Mol Imaging 33:724–729. https://doi.org/10.1007/s00259-005-0029-7
Mori T, Ito H, Harada M et al (2020) Multi-delay arterial spin labeling brain magnetic resonance imaging study for pediatric autism. Brain Dev 42:315–321. https://doi.org/10.1016/j.braindev.2020.01.007
Im K, Ahtam B, Haehn D et al (2016) Altered structural brain networks in tuberous sclerosis complex. Cereb Cortex 26:2046–2058. https://doi.org/10.1093/cercor/bhv026
Tsai J-D, Ho M-C, Lee H-Y et al (2021) Disrupted white matter connectivity and organization of brain structural connectomes in tuberous sclerosis complex patients with neuropsychiatric disorders using diffusion tensor imaging. MAGMA 34:189–200. https://doi.org/10.1007/s10334-020-00870-4
Funding
This study was supported by a research grant from the French Society of Radiology (SFR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Pr Nathalie Boddaert.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this was a retrospective study using anonymized data.
Ethics approval
Institutional Review Board approval was not required because this was a retrospective study using anonymized data.
Methodology
-
retrospective
-
observational
-
performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1923 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rutten, C., Fillon, L., Kuchenbuch, M. et al. The longitudinal evolution of cerebral blood flow in children with tuberous sclerosis assessed by arterial spin labeling magnetic resonance imaging may be related to cognitive performance. Eur Radiol 33, 196–206 (2023). https://doi.org/10.1007/s00330-022-09036-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09036-3