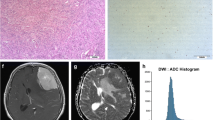
Abstract
Objective
To investigate the value of histogram analysis of T1 mapping and diffusion-weighted imaging (DWI) in predicting the grade, subtype, and proliferative activity of meningioma.
Methods
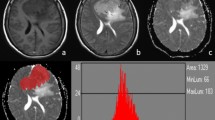
This prospective study comprised 69 meningioma patients who underwent preoperative MRI including T1 mapping and DWI. The histogram metrics, including mean, median, maximum, minimum, 10th percentiles (C10), 90th percentiles (C90), kurtosis, skewness, and variance, of T1 and apparent diffusion coefficient (ADC) values were extracted from the whole tumour and peritumoural oedema using FeAture Explorer. The Mann-Whitney U test was used for comparison between low- and high-grade tumours. Receiver operating characteristic (ROC) curve and logistic regression analyses were performed to identify the differential diagnostic performance. The Kruskal-Wallis test was used to further classify meningioma subtypes. Spearman’s rank correlation coefficients were calculated to analyse the correlations between histogram parameters and Ki-67 expression.
Results
High-grade meningiomas showed significantly higher mean, maximum, C90, and variance of T1 (p = 0.001–0.009), lower minimum, and C10 of ADC (p = 0.013–0.028), compared to low-grade meningiomas. For all histogram parameters, the highest individual distinctive power was T1 C90 with an AUC of 0.805. The best diagnostic accuracy was obtained by combining the T1 C90 and ADC C10 with an AUC of 0.864. The histogram parameters differentiated 4/6 pairs of subtype pairs. Significant correlations were identified between Ki-67 and histogram parameters of T1 (C90, mean) and ADC (C10, kurtosis, variance).
Conclusion
T1 and ADC histogram parameters may represent an in vivo biomarker for predicting the grade, subtype, and proliferative activity of meningioma.
Key Points
• The histogram parameter based on T1 mapping and DWI is useful to preoperatively evaluate the grade, subtype, and proliferative activity of meningioma.
• The combination of T1 C90 and ADC C10 showed the best performance for differentiating low- and high-grade meningiomas.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUCs:
-
Area under the receiver operating characteristic curves
- C10:
-
10th percentile
- C90:
-
90th percentile
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- DWI:
-
Diffusion-weighted imaging
- ECM:
-
Extracellular matrix
- FSPGR:
-
Fast-spoiled gradient recalled
- GRE:
-
Gradient recalled echo
- HGMs:
-
High-grade meningiomas
- ICC:
-
Intraclass correlation coefficient
- IR-FSE:
-
Inversion recovery fast spin echo
- LGMs:
-
Low-grade meningiomas
- LI:
-
Labelling index
- MS:
-
Multiple sclerosis
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- WHO:
-
World Health Organization
References
Shibuya M (2015) Pathology and molecular genetics of meningioma: recent advances. Neurol Med Chir (Tokyo) 55:14–27
Louis D, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5:1045–1054
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) Corrigendum to: CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa269
Banan R, Abbetmeier-Basse M, Hong B et al (2021) The prognostic significance of clinicopathological features in meningiomas: microscopic brain invasion can predict patient outcome in otherwise benign meningiomas. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12700
Goldbrunner R, Stavrinou P, Jenkinson M et al (2021) EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol 23:1821–1834
Kashimura H, Inoue T, Ogasawara K et al (2007) Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg 107:784–787
Swiderska-Chadaj Z, Markiewicz T, Grala B, Lorent M (2016) Content-based analysis of Ki-67 stained meningioma specimens for automatic hot-spot selection. Diagn Pathol 11:93
Marciscano A, Stemmer-Rachamimov A, Niemierko A et al (2016) Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg 124:106–114
Lin L, Xue Y, Duan Q et al (2019) Grading meningiomas using mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging. Clin Radiol 74:651.e615–651.e623
Lin L, Bhawana R, Xue Y et al (2018) Comparative analysis of diffusional kurtosis imaging, diffusion tensor imaging, and diffusion-weighted imaging in grading and assessing cellular proliferation of meningiomas. AJNR Am J Neuroradiol 39:1032–1038
Yu H, Wen X, Wu P et al (2019) Can amide proton transfer-weighted imaging differentiate tumor grade and predict Ki-67 proliferation status of meningioma? Eur Radiol 29:5298–5306
Parsai C, O'Hanlon R, Prasad S, Mohiaddin R (2012) Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magnet Resonance : Official J Soc Cardiovasc Magnet Resonance 14:54
Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S (2016) Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 71:768–778
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18:89
Aherne E, Chow K, Carr J (2020) Cardiac T1 mapping: techniques and applications. J Magn Reson Imaging 51:1336–1356
Fernandes JL, Rochitte CE (2015) T1 mapping: technique and applications. Magn Reson Imaging Clin N Am 23:25–34
Child N, Suna G, Dabir D et al (2018) Comparison of MOLLI, shMOLLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur Heart J Cardiovasc Imaging 19:768–776
Ramsahye H, He H, Feng X, Li S, Xiong J (2013) Central neurocytoma: radiological and clinico-pathological findings in 18 patients and one additional MRS case. J Neuroradiol 40:101–111
Svolos P, Tsolaki E, Kapsalaki E et al (2013) Investigating brain tumor differentiation with diffusion and perfusion metrics at 3T MRI using pattern recognition techniques. Magn Reson Imaging 31:1567–1577
El-Ali A, Agarwal V, Thomas A, Hamilton R, Filippi C (2019) Clinical metric for differentiating intracranial hemangiopericytomas from meningiomas using diffusion weighted MRI. Clin Imaging 54:1–5
Atalay B, Ediz S, Ozbay N (2020) Apparent diffusion coefficient in predicting the preoperative grade of meningiomas. J Coll Physicians Surg Pak 30:1126–1132
Meyer H, Wienke A, Surov A (2020) ADC values of benign and high grade meningiomas and associations with tumor cellularity and proliferation - a systematic review and meta-analysis. J Neurol Sci 415:116975
Xiaoai K, Qing Z, Lei H, Junlin Z (2020) Differentiating microcystic meningioma from atypical meningioma using diffusion-weighted imaging. Neuroradiology 62:601–607
Bozdağ M, Er A, Ekmekçi S (2021) Association of apparent diffusion coefficient with Ki-67 proliferation index, progesterone-receptor status and various histopathological parameters, and its utility in predicting the high grade in meningiomas. Acta Radiol 62:401–413
Yiping L, Kawai S, Jianbo W, Li L, Daoying G, Bo Y (2017) Evaluation parameters between intra-voxel incoherent motion and diffusion-weighted imaging in grading and differentiating histological subtypes of meningioma: a prospective pilot study. J Neurol Sci 372:60–69
Zhang S, Chiang G, Knapp J et al (2020) Grading meningiomas utilizing multiparametric MRI with inclusion of susceptibility weighted imaging and quantitative susceptibility mapping. J Neuroradiol = J Neuroradiol 47:272–277
Ahn SJ, Choi SH, Kim YJ et al (2012) Histogram analysis of apparent diffusion coefficient map of standard and high B-value diffusion MR imaging in head and neck squamous cell carcinoma: a correlation study with histological grade. Acad Radiol 19:1233–1240
Wang S, Kim S, Zhang Y et al (2012) Determination of grade and subtype of meningiomas by using histogram analysis of diffusion-tensor imaging metrics. Radiology 262:584–592
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Liu HS, Chiang SW, Chung HW et al (2018) Histogram analysis of T2*-based pharmacokinetic imaging in cerebral glioma grading. Comput Methods Prog Biomed 155:19–27
Murayama K, Nishiyama Y, Hirose Y et al (2018) Differentiating between central nervous system lymphoma and high-grade glioma using dynamic susceptibility contrast and dynamic contrast-enhanced MR imaging with histogram analysis. Magn Reson Med Sci 17:42–49
Liu P, Chen L, Wang QX et al (2020) Histogram analysis of T2 mapping for detecting early involvement of extraocular muscles in patients with thyroid-associated ophthalmopathy. Sci Rep 10:19445
Li D, Cui Y, Hou L et al (2021) Diffusion kurtosis imaging-derived histogram metrics for prediction of resistance to neoadjuvant chemoradiotherapy in rectal adenocarcinoma: preliminary findings. Eur J Radiol 144:109963
Xie T, Zhao Q, Fu C, Grimm R, Gu Y, Peng W (2021) Improved value of whole-lesion histogram analysis on DCE parametric maps for diagnosing small breast cancer (≤ 1 cm). Eur Radiol. https://doi.org/10.1007/s00330-021-08244-7
Barral J, Gudmundson E, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura D (2010) A robust methodology for in vivo T1 mapping. Magn Reson Med 64:1057–1067
Just M, Thelen M (1988) Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology 169:779–785
Komiyama M, Yagura H, Baba M et al (1987) MR imaging: possibility of tissue characterization of brain tumors using T1 and T2 values. AJNR Am J Neuroradiol 8:65–70
Larsson C, Kleppestø M, Grothe I, Vardal J, Bjørnerud A (2015) T1 in high-grade glioma and the influence of different measurement strategies on parameter estimations in DCE-MRI. J Magn Reson Imaging 42:97–104
Andersen C, Astrup J, Gyldensted C (1994) Quantitative MR analysis of glucocorticoid effects on peritumoural oedema associated with intracranial meningiomas and metastases. J Comput Assist Tomogr 18:509–518
Wang B, Zhang Y, Zhao B et al (2018) Postcontrast T1 mapping for differential diagnosis of recurrence and radionecrosis after gamma knife radiosurgery for brain metastasis. AJNR Am J Neuroradiol 39:1025–1031
Vrenken H, Geurts J, Knol D et al (2006) Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology 240:811–820
van Walderveen M, van Schijndel R, Pouwels P, Polman C, Barkhof F (2003) Multislice T1 relaxation time measurements in the brain using IR-EPI: reproducibility, normal values, and histogram analysis in patients with multiple sclerosis. J Magn Reson Imaging 18:656–664
Griffin C, Dehmeshki J, Chard D et al (2002) T1 histograms of normal-appearing brain tissue are abnormal in early relapsing-remitting multiple sclerosis. Mult Scler 8:211–216
Tan Y, Xu J, Chen R et al (2018) Use of T relaxation time in rotating frame (T ρ) and apparent diffusion coefficient to estimate cerebral stroke evolution. J Magnet Resonance Imaging : JMRI 48:1247–1254
Ayerbe J, Lobato RD, de la Cruz J et al (1999) Risk factors predicting recurrence in patients operated on for intracranialmeningioma. A multivariate analysis. Acta Neurochir (Wien) 141:921–932
Takahashi JA, Ueba T, Hashimoto N, Nakashima Y, Katsuki N (2004) The combination of mitotic and Ki-67 indices as a useful method for predicting short-term recurrence of meningiomas. Surg Neurol 61(149-155):discussion 155-146
Nagar VA, Ye JR, Ng WH et al (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29:1147–1152
Okuducu AF, Zils U, Michaelis SA, Michaelides S, von Deimling A (2006) Ets-1 is up-regulated together with its target gene products matrix metalloproteinase-2 and matrix metalloproteinase-9 in atypical and anaplastic meningiomas. Histopathology 48:836–845
Piechnik SK, Jerosch-Herold M (2018) Myocardial T1 mapping and extracellular volume quantification: an overview of technical and biological confounders. Int J Card Imaging 34:3–14
Adams LC, Ralla B, Jurmeister P et al (2019) Native T1 mapping as an in vivo biomarker for the identification of higher-grade renal cell carcinoma: correlation with histopathological findings. Invest Radiol 54:118–128
Ma R, Geng Y, Gan L et al (2021) Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy. Endocrine. https://doi.org/10.1007/s12020-021-02873-0
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Surov A, Gottschling S, Mawrin C et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. Transl Oncol 8:517–523
Sanverdi SE, Ozgen B, Oguz KK et al (2012) Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol 81:2389–2395
Park YW, Kim S, Ahn SS et al (2020) Magnetic resonance imaging-based 3-dimensional fractal dimension and lacunarity analyses may predict the meningioma grade. Eur Radiol 30:4615–4622
Hsu C, Pai C, Kao H, Hsueh C, Hsu W, Lo C (2010) Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci 17:584–587
Choi Y, Kim S, Youn I, Kang B, Park W, Lee A (2017) Rim sign and histogram analysis of apparent diffusion coefficient values on diffusion-weighted MRI in triple-negative breast cancer: comparison with ER-positive subtype. PLoS One 12:e0177903
Surov A, Ginat D, Lim T et al (2018) Histogram analysis parameters apparent diffusion coefficient for distinguishing high and low-grade meningiomas: a multicenter study. Transl Oncol 11:1074–1079
Gihr G, Horvath-Rizea D, Garnov N et al (2018) Diffusion profiling via a histogram approach distinguishes low-grade from high-grade meningiomas, can reflect the respective proliferative potential and progesterone receptor status. Mol Imaging Biol 20:632–640
Bohara M, Nakajo M, Kamimura K et al (2020) Histological grade of meningioma: prediction by intravoxel incoherent motion histogram parameters. Acad Radiol 27:342–353
Lu S, Kim S, Kim N, Kim H, Choi C, Lim Y (2015) Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol 204:827–834
He W, Xiao X, Li X et al (2019) Whole-tumor histogram analysis of apparent diffusion coefficient in differentiating intracranial solitary fibrous tumor/hemangiopericytoma from angiomatous meningioma. Eur J Radiol 112:186–191
Lu Y, Liu L, Luan S, Xiong J, Geng D, Yin B (2019) The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: an attempt using decision tree and decision forest. Eur Radiol 29:1318–1328
Li X, Miao Y, Han L et al (2019) Meningioma grading using conventional MRI histogram analysis based on 3D tumor measurement. Eur J Radiol 110:45–53
Pond J, Morgan T, Hatanpaa K, Yetkin Z, Mickey B, Mendelsohn D (2015) Chordoid meningioma: differentiating a rare World Health Organization grade II tumor from other meningioma histologic subtypes using MRI. AJNR Am J Neuroradiol 36:1253–1258
King AD, Chow KK, Yu KH et al (2013) Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology 266:531–538
Cho GY, Moy L, Kim SG et al (2016) Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol 26:2547–2558
Westfall PH (2014) Kurtosis as Peakedness, 1905 - 2014. R.I.P. Am Stat 68:191-195
Del Gobbo A, Pellegrinelli A, Gaudioso G et al (2016) Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology 68:746–751
Becker A, Wagner M, Wurnig M, Boss A (2017) Diffusion-weighted imaging of the abdomen: impact of b-values on texture analysis features. NMR Biomed https://doi.org/10.1002/nbm.3669
Acknowledgements
We acknowledge PuYeh Wu from GE Healthcare for the technical support.
Funding
This study has received funding from the Joint Funds for the Innovation of Science and Technology, Fujian province (Grant number: 2018Y9044) and Fujian Provincial Health Technology Project (grant number: 2020GGA039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Lin Lin, MD, PhD, Fujian Medical University Union Hospital.
Conflict of interest
One of the authors of this manuscript (PuYeh Wu) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 314 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, T., Jiang, R., Zheng, L. et al. T1 and ADC histogram parameters may be an in vivo biomarker for predicting the grade, subtype, and proliferative activity of meningioma. Eur Radiol 33, 258–269 (2023). https://doi.org/10.1007/s00330-022-09026-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09026-5