Abstract
Objectives
This study aimed to develop and validate a nomogram based on extracellular volume (ECV) derived from computed tomography (CT) for predicting post-hepatectomy liver failure (PHLF) in patients with resectable hepatocellular carcinoma (HCC).
Methods
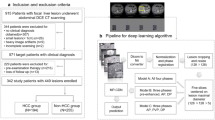
A total of 202 patients with resectable HCC from two hospitals were enrolled and underwent multiphasic contrast-enhanced CT before surgery. One hundred twenty-one patients from our hospital and 81 patients from another hospital were assigned to the training cohort and the validation cohort, respectively. CT–derived ECV was measured using nonenhanced and equilibrium-phase-enhanced CT images. The nomogram was developed with independent predictors of PHLF. Predictive performance and calibration were assessed by receiver operator characteristic (ROC) analysis and Hosmer–Lemeshow test, respectively. The Delong test was used to compare the areas under the curve (AUCs).
Results
CT–derived ECV had a strong correlation with the postoperative pathological fibrosis stage of the background liver (p < 0.001, r = 0.591). The nomogram combining CT–derived ECV, serum albumin (Alb), and serum total bilirubin (Tbil) obtained higher AUCs than the albumin–bilirubin (ALBI) score for predicting PHLF in both the training cohort (0.828 vs. 0.708; p = 0.004) and the validation cohort (0.821 vs. 0.630; p < 0.001). The nomogram showed satisfactory goodness of fit for PHLF prediction in the training and validation cohorts (p = 0.621 and 0.697, respectively).
Conclusions
The nomogram contributes to the preoperative prediction of PHLF in patients with resectable HCC.
Key Points
• CT–derived ECV had a strong correlation with the postoperative pathological fibrosis stage of the background liver.
• CT–derived ECV was an independent predictor of PHLF in patients with resectable HCC.
• The nomogram based on CT–derived ECV showed a superior prediction efficacy than that of clinical models (including Child–Pugh stage, MELD score, and ALBI score).




Similar content being viewed by others
Abbreviations
- Alb:
-
Albumin
- ALBI:
-
Albumin–bilirubin
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- ECV:
-
Extracellular volume
- EP:
-
Equilibrium phase
- HCC:
-
Hepatocellular carcinoma
- Hct:
-
Hematocrit
- ISGLS:
-
International Study Group of Liver Surgery
- MELD:
-
Model for End-Stage Liver Disease
- PHLF:
-
Post-hepatectomy liver failure
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- Tbil:
-
Total bilirubin
References
Dhir M, Melin AA, Douaiher J et al (2016) A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg 263:1112–1125
Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J (1999) Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 229:210–215
Mullen JT, Ribero D, Reddy SK et al (2007) Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 204:854–862 discussion 862-854
Rahbari NN, Garden OJ, Padbury R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Gilg S, Sandstrom P, Rizell M et al (2018) The impact of post-hepatectomy liver failure on mortality: a population-based study. Scand J Gastroenterol 53:1335–1339
Soreide JA, Deshpande R (2021) Post hepatectomy liver failure (PHLF) - recent advances in prevention and clinical management. Eur J Surg Oncol 47:216–224
Tsujita Y, Sofue K, Komatsu S et al (2020) Prediction of post-hepatectomy liver failure using gadoxetic acid-enhanced magnetic resonance imaging for hepatocellular carcinoma with portal vein invasion. Eur J Radiol 130:109189
van den Broek MA, Olde Damink SW, Dejong CH et al (2008) Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 28:767–780
Zou H, Yang X, Li QL, Zhou QX, Xiong L, Wen Y (2018) A comparative study of albumin-bilirubin score with Child-Pugh score, Model for End-Stage Liver Disease score and indocyanine green R15 in predicting posthepatectomy liver failure for hepatocellular carcinoma patients. Dig Dis 36:236–243
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea (2019) 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 20:1042–1113
Varenika V, Fu Y, Maher JJ et al (2013) Hepatic fibrosis: evaluation with semiquantitative contrast-enhanced CT. Radiology 266:151–158
Bandula S, Punwani S, Rosenberg WM et al (2015) Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143
Shinagawa Y, Sakamoto K, Sato K, Ito E, Urakawa H, Yoshimitsu K (2018) Usefulness of new subtraction algorithm in estimating degree of liver fibrosis by calculating extracellular volume fraction obtained from routine liver CT protocol equilibrium phase data: preliminary experience. Eur J Radiol 103:99–104
Bak S, Kim JE, Bae K et al (2020) Quantification of liver extracellular volume using dual-energy CT: utility for prediction of liver-related events in cirrhosis. Eur Radiol 30:5317–5326
Yoon JH, Lee JM, Kim JH et al (2021) Hepatic fibrosis grading with extracellular volume fraction from iodine mapping in spectral liver CT. Eur J Radiol 137:109604
Guo SL, Su LN, Zhai YN et al (2017) The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast-enhanced liver CT for staging liver fibrosis. Clin Radiol 72:242–246
Zhang Z, Ouyang G, Wang P et al (2021) Safe standard remnant liver volume after hepatectomy in HCC patients in different stages of hepatic fibrosis. BMC Surg 21:57
Feng JW, Qu Z, Wu BQ, Sun DL, Jiang Y (2019) The preoperative fibrosis score 4 predicts posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann Hepatol 18:701–707
Hu H, Han H, Han XK, Wang WP, Ding H (2018) Nomogram for individualised prediction of liver failure risk after hepatectomy in patients with resectable hepatocellular carcinoma: the evidence from ultrasound data. Eur Radiol 28:877–885
Reddy SK, Barbas AS, Turley RS et al (2011) A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 13:494–502
Motoyama H, Kobayashi A, Yokoyama T et al (2014) Liver failure after hepatocellular carcinoma surgery. Langenbecks Arch Surg 399:1047–1055
Kong FH, Miao XY, Zou H et al (2019) End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma. World J Clin Cases 7:3734–3741
Kubota K, Makuuchi M, Kusaka K et al (1997) Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 26:1176–1181
Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF (2011) Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg 35:2073–2082
Shen YN, Tang TY, Yao WY et al (2019) A nomogram for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma: a retrospective study. Medicine (Baltimore) 98:e18490
Cescon M, Colecchia A, Cucchetti A et al (2012) Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 256:706–712 discussion 712-703
Shen Y, Zhou C, Zhu G et al (2017) Liver stiffness assessed by shear wave elastography predicts postoperative liver failure in patients with hepatocellular carcinoma. J Gastrointest Surg 21:1471–1479
Castera L, Foucher J, Bernard PH et al (2010) Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51:828–835
Arena U, Vizzutti F, Corti G et al (2008) Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 47:380–384
Liang XE, Chen YP, Zhang Q, Dai L, Zhu YF, Hou JL (2011) Dynamic evaluation of liver stiffness measurement to improve diagnostic accuracy of liver cirrhosis in patients with chronic hepatitis B acute exacerbation. J Viral Hepat 18:884–891
Acknowledgements
We would like thank all the participants of this study.
Funding
This study has received funding from the National Natural Science Foundation of China (Grant No. 82071883), the combination projects of medicine and engineering of the Fundamental Research Funds for the Central Universities in 2019 (Project No. 2019CDYGYB008), and the Chongqing key medical research project of a combination of science and medicine (Grant No. 2021MSXM077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jiuquan Zhang, from the Department of Radiology, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, People’s Republic of China, email: zhangjq_radiol@foxmail.com.
Conflict of interest
One of the authors (Xiaoyue Zhang) of this manuscript is an employee of Siemens Healthineers. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approvals of the two participating hospitals were obtained.
Methodology
retrospective
diagnostic or prognostic study
multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure S1.
Handcrafted ROIs diagram and calculation process of the CT-derived ECV. A 39-year-old man with HCC, underwent a major hepatectomy (resection of right lobe and caudate lobe of liver) and with a known of Hct value was 47%. In the preoperative baseline nonenhanced (A-C) and EP enhanced (D-F) images, handcrafted ROIs were drawn along the margins of the future remnant liver, and circular ROIs were drawn in the abdominal aorta on the same plane. ROIs were placed on three cross-sections, including intrahepatic inferior vena cava confluence (A, D), portal hilum (B, E) and gallbladder fossa (C, F). The ECV values of the section at intrahepatic inferior vena cava confluence (ECV1), portal hilum (ECV2) and gallbladder fossa (ECV3) were calculated by the formula shown in the figure, respectively. Then the CT-derived ECV for the patient (ECV = 33.00) was the mean value of ECV1, ECV2 and ECV3. ROI, region of interest; CT, computed tomography; ECV, extracellular volume; HCC, hepatocellular carcinoma; EP, equilibrium phase; Hct, hematocrit. (PNG 9760 kb)
Figure S2.
Bland–Altman plots of the CT-derived ECV differences measured by radiologist 1 with an interval of 4 weeks (A, C), and radiologist 1/2 (B, D). A/B and C/D were for the training and validation cohort, respectively. These Bland–Altman plots showed good agreement. CT, computed tomography; ECV, extracellular volume. (PNG 265 kb)
Figure S3.
Two examples of clinical application of our nomogram. (A) a 51-year-old man with resectable HCC, the point of CT-derived ECV (22.00), serum Alb (38.00 g/L) and serum Tbil (18.98 μmol/L) were indicated by orange dotted lines, then the total point of this patient was indicated by red dotted line, indicating that the risk of PHLF in this patient was much less than 35%. (B) a 64-year-old man with resectable HCC, the point of CT-derived ECV (27.01), serum Alb (33.61 g/L) and serum Tbil (131.13 μmol/L) were indicated by orange dotted lines, then the total point of this patient was indicated by red dotted line, indicating that the risk of PHLF in this patient was exceeded 85%. Therefore, this patient should consider a new strategy for clinical treatment. HCC, hepatocellular carcinoma; CT, computed tomography; ECV, extracellular volume; Alb, albumin; Tbil, total bilirubin; PHLF, post-hepatectomy liver failure. (PNG 543 kb)
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Peng, Y., Shen, H., Tang, H. et al. Nomogram based on CT–derived extracellular volume for the prediction of post-hepatectomy liver failure in patients with resectable hepatocellular carcinoma. Eur Radiol 32, 8529–8539 (2022). https://doi.org/10.1007/s00330-022-08917-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08917-x