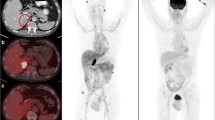
Abstract
Objectives
To associate the early change in DL-CT parameters and HU with survival outcomes and treatment response in patients with metastatic renal cell carcinoma (mRCC).
Methods
DL-CT scans were performed at baseline and after 1 month of checkpoint immunotherapy or tyrosine kinase inhibitor therapy. Scans were reconstructed to conventional CT and DL-CT series, and used for assessment of HU, iodine concentration (IC), and the effective atomic number (Zeffective) in the combined RECISTv.1.1 target lesions. The relative changes, defined as ΔIC(combined), ΔZeffective(combined), and ΔHU(combined), were associated with progression-free survival (PFS), overall survival (OS), and objective response rate (ORR). The reduction in the sum of diameters of target lesions ≥ 30% after 1 month was associated with OS, PFS, and ORR.
Results
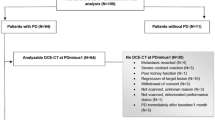
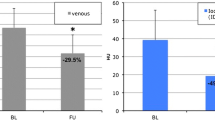
Overall, 115 and 104 mRCC patients were included at baseline and 1 month, respectively. Median IC(combined) decreased from 2.3 to 1.2 mg/ml (p < 0.001), Zeffective(combined) from 8.5 to 8.0 (p < 0.001), and HU(combined) from 86.0 to 64.00 HU (p < 0.001). After multivariate adjustments, the largest reductions in ΔIC(combined) (HR 0.47, 95% CI: 0.24–0.94, p = 0.033) and ΔZeffective(combined) (HR = 0.43, 95% CI: 0.21–0.87, p = 0.019) were associated with favorable OS; the largest reduction in ΔZeffective(combined) was associated with higher response (OR = 2.79, 95% CI: 1.12–6.94, p = 0.027). The largest reduction in ΔHU(combined) was solely associated with OS in univariate analysis (HR 0.45, 95% CI: 0.23–0.91). Reduction in SOD ≥ 30% at 1 month was not associated with outcomes (p > 0.075).
Conclusions
Early reductions at 1 month in ΔIC(combined) and ΔZeffective(combined) are associated with favorable outcomes in patients with mRCC. This information may reassure physicians and patients about treatment strategy.
Key Points
• Early reductions following 1 month of therapy in spectral dual-layer detector CT-derived iodine concentration and the effective atomic number (Z effective ) are independent biomarkers for better overall survival in patients with metastatic renal cell carcinoma.
• Early reduction after 1 month of therapy in the effective atomic number (Z effective ) is an independent imaging biomarker for better treatment response metastatic renal cell carcinoma.





Similar content being viewed by others
Abbreviations
- ccRCC:
-
Clear cell RCC
- CPI:
-
Checkpoint immunotherapy
- DL-CT:
-
Spectral dual-layer computed tomography
- mRCC:
-
Metastatic renal cell carcinoma
- nccRCC:
-
Non-clear cell RCC
- RECIST v1.1:
-
Response Evaluation Criteria in Solid Tumors version 1.1
- TKI:
-
Tyrosine kinase inhibitors
- VOI:
-
Volume of interest
- ΔHU(combined):
-
The relative change from baseline to 1 month in the median Hounsfield units (HU) measured in the combined RECIST v1.1-defined target lesions
- ΔIC(combined):
-
The relative change from baseline to 1 month in the median iodine concentration (mg/ml) measured in the combined RECIST v1.1-defined target lesions
- ΔZeffective(combined):
-
The relative change from baseline to 1 month in the median effective atomic number (ΔZeffective) measured in the combined RECIST v1.1-defined target lesions
References
Hsieh JJ, Purdue MP, Signoretti S et al (2017) Renal cell carcinoma. Nat Rev Dis Primers 3:17009. https://doi.org/10.1038/nrdp.2017.9
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol 70:93–105. https://doi.org/10.1016/j.eururo.2016.02.029
Cancer Genome Atlas Research Network (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 499:43–49. https://doi.org/10.1038/nature12222
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S (2016) Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol 34:1081–1086. https://doi.org/10.1007/s00345-016-1773-y
Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P (2006) Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol 24:3101–3106. https://doi.org/10.1200/JCO.2005.04.8280
Gerwing M, Herrmann K, Helfen A et al (2019) The beginning of the end for conventional RECIST - novel therapies require novel imaging approaches. Nat Rev Clin Oncol 16:442–458. https://doi.org/10.1038/s41571-019-0169-5
Motzer RJ, Penkov K, Haanen J et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103–1115. https://doi.org/10.1056/NEJMoa1816047
Powles T, Plimack ER, Soulières D et al (2020) Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21:1563–1573. https://doi.org/10.1016/S1470-2045(20)30436-8
Choueiri TK, Escudier B, Powles T et al (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917–927. https://doi.org/10.1016/S1470-2045(16)30107-3
Albiges L, Tannir NM, Burotto M et al (2020) Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079. https://doi.org/10.1136/esmoopen-2020-001079
Choueiri TK, Powles T, Burotto M et al (2021) Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829–841. https://doi.org/10.1056/NEJMoa2026982
Motzer R, Alekseev B, Rha SY et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289–1300. https://doi.org/10.1056/NEJMoa2035716
Motzer RJ, Escudier B, George S et al (2020) Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 126:4156–4416. https://doi.org/10.1002/cncr.33033
McDermott DF, Lee JL, Bjarnason GA et al (2021) Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol 39:1029–1039. https://doi.org/10.1200/JCO.20.02363
McDermott DF, Lee JL, Ziobro M (2021) Open-label, single-arm, phase ii study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol 39:1029–1039. https://doi.org/10.1200/jco.20.02365
Heng DY, MacKenzie MJ, Vaishampayan UN et al (2012) Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol 23:1549–1555. https://doi.org/10.1093/annonc/mdr533
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Schwartz LH, Litiere S, de Vries E et al (2016) RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081
Simons D, Kachelriess M, Schlemmer HP (2014) Recent developments of dual-energy CT in oncology. Eur Radiol 24:930–939. https://doi.org/10.1007/s00330-013-3087-4
Andersen MB, Ebbesen D, Thygesen J, Kruis M, Rasmussen F (2020) Impact of spectral body imaging in patients suspected for occult cancer: a prospective study of 503 patients. Eur Radiol 30:5539–5550. https://doi.org/10.1007/s00330-020-06878-7
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 276:637–653. https://doi.org/10.1148/radiol.2015142631
McCollough CH (2020) Methods for spectral CT imaging. In: Samei E, Pelc N (eds) Computed tomography. Springer, Cham, pp 223–242. https://doi.org/10.1007/978-3-030-26957-9_12
McCollough CH, Boedeker K, Cody D (2020) Principles and applications of multienergy CT: report of AAPM Task Group 291. Med Phys 47:e881–e912. https://doi.org/10.1002/mp.14157
Rassouli N, Etesami M, Dhanantwari A, Rajiah P (2017) Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging 8:589–598. https://doi.org/10.1007/s13244-017-0571-4
Sodickson AD, Keraliya A, Czakowski B, Primak A, Wortman J, Uyeda JW (2021) Dual energy CT in clinical routine: how it works and how it adds value. Emerg Radiol 28:103–117. https://doi.org/10.1007/s10140-020-01785-2
Thaiss WM, Haberland U, Kaufmann S et al (2016) Iodine concentration as a perfusion surrogate marker in oncology: further elucidation of the underlying mechanisms using volume perfusion CT with 80 kVp. Eur Radiol 26:2929–2936. https://doi.org/10.1007/s00330-015-4154-9
Mileto A, Marin D, Alfaro-Cordoba M et al (2014) Iodine quantification to distinguish clear cell from papillary renal cell carcinoma at dual-energy multidetector CT: a multireader diagnostic performance study. Radiology. 273:813–820. https://doi.org/10.1148/radiol.14140171
Marcon J, Graser A, Horst D et al (2020) Papillary vs clear cell renal cell carcinoma. Differentiation and grading by iodine concentration using DECT-correlation with microvascular density. Eur Radiol 30:1–10. https://doi.org/10.1007/s00330-019-06298-2
Udare A, Walker D, Krishna S et al (2020) Characterization of clear cell renal cell carcinoma and other renal tumors: evaluation of dual-energy CT using material-specific iodine and fat imaging. Eur Radiol 30:2091–2102. https://doi.org/10.1007/s00330-019-06590-1
Drljevic-Nielsen A, Donskov F, Mains JR et al (2021) Prognostic utility of parameters derived from pretreatment dual-layer spectral-detector ct in patients with metastatic renal cell carcinoma. AJR Am J Roentgenol 218:1–11. https://doi.org/10.2214/ajr.21.26911
Hellbach K, Sterzik A, Sommer W et al (2017) Dual energy CT allows for improved characterization of response to antiangiogenic treatment in patients with metastatic renal cell cancer. Eur Radiol 27:2532–2537. https://doi.org/10.1007/s00330-016-4597-7
Heng DY, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799. https://doi.org/10.1200/JCO.2008.21.4809
Turajlic S, Xu H, Litchfield K et al (2018) Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell. 173:581–594.e12. https://doi.org/10.1016/j.cell.2018.03.057
van Ommen F, de Jong H, Dankbaar JW, Bennink E, Leiner T, Schilham AMR (2019) Dose of CT protocols acquired in clinical routine using a dual-layer detector CT scanner: a preliminary report. Eur J Radiol 112:65–71. https://doi.org/10.1016/j.ejrad.2019.01.011
Mileto A, Allen BC, Pietryga JA (2017) Characterization of incidental renal mass with dual-energy CT: diagnostic accuracy of effective atomic number maps for discriminating nonenhancing cysts from enhancing masses. 209:W221-230. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.16.17325
Main JR, Donskov F, Pedersen EM, Madsen HHT, Rasmussen F (2014) Dynamic contrast-enhanced computed tomography as a potential biomarker in patients with metastatic renal cell carcinoma: preliminary results from the Danish Renal Cancer Group Study-1. Invest Radiol 49:601–607. https://doi.org/10.1097/RLI.0000000000000058
Mains JR, Donskov F, Pedersen EM, Madsen HHT, Rasmussen F (2017) Dynamic contrast-enhanced computed tomography-derived blood volume and blood flow correlate with patient outcome in metastatic renal cell carcinoma. Invest Radiol 52:103–110. https://doi.org/10.1097/RLI.0000000000000315
Mains JR, Donskov F, Pedersen EM et al (2018) Use of patient outcome endpoints to identify the best functional CT imaging parameters in metastatic renal cell carcinoma patients. Br J Radiol 91:20160795. https://doi.org/10.1259/bjr.20160795
Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN (2021) Cardiotoxic effects of angiogenesis inhibitors. Clin Sci 135:71–100. https://doi.org/10.1042/CS20200305
Zhang JC, Chen WD, Alvarez JB et al (2018) Cancer immune checkpoint blockade therapy and its associated autoimmune cardiotoxicity. Acta Pharmacol Sin 39:1693–1698. https://doi.org/10.1038/s41401-018-0062-2
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 256:32–61. https://doi.org/10.1148/radiol.10090908
Acknowledgements
We wish to acknowledge Ipsen for supporting the study financially.
Funding
This study has received funding by Ipsen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Finn Rasmussen.
Conflict of interest
Aska Drljevic-Nielsen reports a research grant from Ipsen and teaching fees from Philips. Frede Donskov reports research grants from Ipsen, Pfizer, MSD, and The Health Research Foundation, Central Denmark Region. Michael Brun Andersen reports teaching fees from Philips and Boehringer Ingelheim. No potential conflicts of interest were disclosed by the other authors.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
The Regional Ethics Committee and Regional Data Protection Agency approved the study. Informed written consent was obtained prior to inclusion.
Study subjects or cohorts overlap
Association of baseline data with patient outcomes in this patient group has been analyzed separately and has been accepted for publication in AJR (currently in press). This is also noted in the methods section of the manuscript.
Methodology
• Prospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drljevic-Nielsen, A., Mains, J.R., Thorup, K. et al. Early reduction in spectral dual-layer detector CT parameters as favorable imaging biomarkers in patients with metastatic renal cell carcinoma. Eur Radiol 32, 7323–7334 (2022). https://doi.org/10.1007/s00330-022-08793-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08793-5