Abstract
Objective
To prospectively determine the value of post-MRI micro-ultrasonography (microUS) in the diagnosis of transition zone (TZ) significant prostate cancer (sPCa).
Patients and methods
Eighty-four consecutive men (66 ± 6.3 years) with a mean PSA level of 10.2 ± 7.4 ng/mL and at least one TZ-PI-RADS > 2 lesion were included. All patients had MRI-directed microUS and biopsy. Sensitivity and specificity of post-MRI microUS to visualize PI-RADS > 2 TZ lesions, the cancer detection rate of TZ-sPCa, and tumor characteristics according to their visibility on microUS were evaluated. Interreader agreement for detecting microUS+ lesions was evaluated using Cohen’s kappa test.
Results
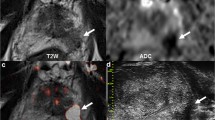
Of the 92 PI-RADS > 2 lesions, 71 (71/92; 77%) were visible on microUS and biopsy was performed without image fusion, which was required for the 21 invisible lesions (21/92; 22.8%). TZ-sPCa detection rate was 51.1% (47/92). Sensitivity and specificity of MRI-directed microUS were 83% (39/47; 95% CI: 69.2–92.4%) and 28.9% (13/45; 95% CI: 16.4–44.3%), on a per-lesion basis and 86.4% (38/45; 95% CI: 72.6–94.8%) and 27.5% (11/40; 95% CI: 14.6–43.9%) on a per-patient basis. Visible tumors on microUS exhibited a larger volume and a lower mean ADC value than non-visible tumors (15.8 ± 5.1 vs. 12.5 ± 3.6 mm and 0.82 ± 1.1 × 103 vs. 0.9 ± 1.4 × 10−3 mm2/s) (p = 0.02). Non-visible tumors showed a heterogeneous non-specific echotexture or were masked by the shadowing caused by corpora amylacea. Interreader agreement was almost perfect (kappa = 0.88; 95% CI: 0.79–0.95). The main limitation is the single-center feature of the study.
Conclusion
MRI-targeted transrectal microUS is effective to detect TZ-sPCa. TRUS-MRI image fusion helps overcome limitations due to TZ tissue heterogeneity.
Key Points
-
microUS can visualize the majority of MRI-detected PI-RADS > 2 TZ lesions (sensitivity = 83%).
-
Interreader agreement of MRI-directed microUS in the detection of TZ lesions appears excellent (kappa = 0.88).
-
In 77% of PI-RADS > 2 TZ lesions, biopsy was performed under microUS visual control. MRI fusion system was only used to overcome limitations due to tissue heterogeneity of benign prostatic hyperplasia.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AFMS:
-
Anterior fibro muscular stroma
- BPH:
-
Benign prostatic hyperplasia
- bp-MRI:
-
Biparametric-MRI
- DWI:
-
Diffusion-weighted imaging
- microUS:
-
Micro-ultrasound
- MRI:
-
Magnetic resonance imaging
- PCa:
-
Prostate cancer
- PI-RADS:
-
Prostate Imaging Reporting and Data System
- PSA:
-
Prostate-specific antigen
- PZ:
-
Peripheral zone
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- sPCa:
-
Significant prostate cancer
- T2WI:
-
T2-weighted images
- TRUS:
-
Transrectal ultrasound
- TZ:
-
Transition zone
References
Won SY, Cho NH, Choi YD, Park SY (2020) Transrectal ultrasound-guided targeted biopsy of transition zone prostate cancer under cognitive registration with prebiopsy MRI and sonographic findings. Clin Radiol 75(157):21–27
Ukimura O, Marien A, Palmer S et al (2015) Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J Urol 33:1669–1676
van de Ven WJM, Sedelaar JPM, van der Leest MMG et al (2016) Visibility of prostate cancer on transrectal ultrasound during fusion with multiparametric magnetic resonance imaging for biopsy. Clin Imaging 40:745–750
Ghai S, Eure G, Fradet V et al (2016) Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol 196:562–569
Abouassaly R, Klein EA, El-Shefai A, Stephenson A (2020) Impact of using 29 MHz high-resolution micro-ultrasound in real-time targeting of transrectal prostate biopsies: initial experience. World J Urol 38:1201–1206
Lughezzani G, Saita A, Lazzeri M et al (2019) Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. Eur Urol Oncol 2:329–332
Cornud F, Lefevre A, Flam T et al (2020) MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: initial results of a single-center study. Eur Radiol 30:4838–4846
Lughezzani G, Maffei D, Saita A et al (2020) Diagnostic accuracy of microultrasound in patients with a suspicion of prostate cancer at magnetic resonance imaging: a single-institutional prospective study. Eur Urol Focus 14(2405-4569):30272–30278
Padhani AR, Barentsz J, Villeirs G et al (2019) PI-RADS steering committee: the PI-RADS multiparametric MRI and MRI-directed biopsy pathway. Radiology 292:464–474
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 76:340–351
Katahira K, Takahara T, Kwee TC et al (2011) Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 21:188–196
Feuerlein S, Davenport MS, Krishnaraj A, Merkle EM, Gupta RT (2015) Computed high b-value diffusion-weighted imaging improves lesion contrast and conspicuity in prostate cancer. Prostate Cancer Prostatic Dis 18:155–160
Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng F-M, Taneja SS (2015) Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR Am J Roentgenol 204:266–272
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol 69:16–40
Ahmed HU, Hu Y, Carter T et al (2011) Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol 186:458–464
Epstein JI, Amin MB, Fine SW et al (2021) The 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch Pathol Lab Med 145:461–493
Benchoufi M, Matzner-Lober E, Molinari N, Jannot A-S, Soyer P (2020) Interobserver agreement issues in radiology. Diagn Interv Imaging 101:639–641
Wiemer L, Hollenbach M, Heckmann R et al (2020) Evolution of targeted prostate biopsy by adding micro-ultrasound to the magnetic resonance imaging pathway. Eur Urol Focus 2405-4569:30188–30187
Klotz L, Lughezzani G, Maffei D et al (2021) Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: a multicenter, prospective analysis. Can Urol Assoc J 15:11–16
Costa DN, Xi Y, Aziz M et al (2019) Prospective inclusion of apparent diffusion coefficients in multiparametric prostate MRI structured reports: discrimination of clinically insignificant and significant cancers. AJR Am J Roentgenol 212:109–116
Polanec SH, Lazar M, Wengert GJ et al (2018) 3D T2-weighted imaging to shorten multiparametric prostate MRI protocols. Eur Radiol 28:1634–1641
Rosenkrantz AB, Neil J, Kong X et al (2010) Prostate cancer: comparison of 3D T2-weighted with conventional 2D T2-weighted imaging for image quality and tumor detection. AJR Am J Roentgenol 194:446–452
Acknowledgements
The authors thank Mrs. Dorothee BOSSIS, PhD, for her manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is F. H. Cornelis.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cornud, F., Lefevre, A., Camparo, P. et al. Post-MRI transrectal micro-ultrasonography of transition zone PI-RADS > 2 lesions for biopsy guidance. Eur Radiol 32, 7504–7512 (2022). https://doi.org/10.1007/s00330-022-08788-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08788-2