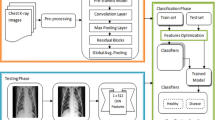
Abstract
Objectives
Chronic obstructive pulmonary disease (COPD) is underdiagnosed globally. The present study aimed to develop weakly supervised deep learning (DL) models that utilize computed tomography (CT) image data for the automated detection and staging of spirometry-defined COPD.
Methods
A large, highly heterogeneous dataset was established, consisting of 1393 participants retrospectively recruited from outpatient, inpatient, and physical examination center settings of four large public hospitals in China. All participants underwent both inspiratory chest CT scans and pulmonary function tests. CT images, spirometry data, demographic information, and clinical information of each participant were collected. An attention-based multi-instance learning (MIL) model for COPD detection was trained using CT scans from 837 participants. External validation of the COPD detection was performed with 620 low-dose CT (LDCT) scans acquired from the National Lung Screening Trial (NLST) cohort. A multi-channel 3D residual network was further developed to categorize GOLD stages among confirmed COPD patients.
Results
The attention-based MIL model used for COPD detection achieved an area under the receiver operating characteristic curve (AUC) of 0.934 (95% CI: 0.903, 0.961) on the internal test set and 0.866 (95% CI: 0.805, 0.928) on the LDCT subset acquired from the NLST. The multi-channel 3D residual network was able to correctly grade 76.4% of COPD patients in the test set (423/553) using the GOLD scale.
Conclusions
The proposed chest CT-DL approach can automatically identify spirometry-defined COPD and categorize patients according to the GOLD scale. As such, this approach may be an effective case-finding tool for COPD diagnosis and staging.
Key Points
• Chronic obstructive pulmonary disease is underdiagnosed globally, particularly in developing countries.
• The proposed chest computed tomography (CT)–based deep learning (DL) approaches could accurately identify spirometry-defined COPD and categorize patients according to the GOLD scale.
• The chest CT-DL approach may be an alternative case-finding tool for COPD identification and evaluation.






Similar content being viewed by others
Change history
23 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00330-022-08693-8
Abbreviations
- %LAA-950:
-
The percentage of lung volume less than or equal to −950 Hounsfield units
- 95% CI:
-
95% confidence interval
- AI:
-
Artificial intelligence
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- DL:
-
Deep learning
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- IQR:
-
Interquartile range
- LDCT:
-
Low-dose computed tomography
- MIL:
-
Multi-instance learning
- NA:
-
Not applicable
- NLST:
-
National Lung Screening Trial
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- SD:
-
Standard deviation
- Yrs:
-
Years
References
Disease GBD, Injury I, Prevalence C (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602
Halpin DMG, Criner GJ, Papi A et al (2021) Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 203:24–36
Jithoo A, Enright PL, Burney P et al (2013) Case-finding options for COPD: results from the Burden of Obstructive Lung Disease study. Eur Respir J 41:548–555
Perez-Padilla R, Thirion-Romero I, Guzman N (2018) Underdiagnosis of chronic obstructive pulmonary disease: should smokers be offered routine spirometry tests? Expert Rev Respir Med 12:83–85
Lamprecht B, Soriano JB, Studnicka M et al (2015) Determinants of underdiagnosis of COPD in national and international surveys. Chest 148:971–985
Wang C, Xu J, Yang L et al (2018) Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 391:1706–1717
Miller MR, Levy ML (2015) Chronic obstructive pulmonary disease: missed diagnosis versus misdiagnosis. BMJ 351:h3021
Estepar RS, Kinney GL, Black-Shinn JL et al (2013) Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med 188:231–239
McDonald ML, Diaz AA, Ross JC et al (2014) Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 11:326–334
Bhatt SP, Washko GR, Hoffman EA et al (2019) Imaging advances in chronic obstructive pulmonary disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study. Am J Respir Crit Care Med 199:286–301
Park J, Hobbs BD, Crapo JD et al (2019) Subtyping COPD using visual and quantitative CT features. Chest. https://doi.org/10.1016/j.chest.2019.06.015
Washko GR, Parraga G (2018) COPD biomarkers and phenotypes: opportunities for better outcomes with precision imaging. Eur Respir J 52
Kauczor HU, Bonomo L, Gaga M et al (2015) ESR/ERS white paper on lung cancer screening. Eur Radiol 25:2519–2531
Lathan C, Frank DA (2013) ACP Journal Club. Review: Low-dose CT screening reduces lung cancer and mortality in current or former smokers. Ann Intern Med 159(JC3)
Chassagnon G, Vakalopolou M, Paragios N, Revel MP (2020) Deep learning: definition and perspectives for thoracic imaging. Eur Radiol 30:2021–2030
Philbrick KA, Yoshida K, Inoue D et al (2018) What does deep learning see? Insights from a classifier trained to predict contrast enhancement phase from CT images. AJR Am J Roentgenol 211:1184–1193
Kermany DS, Goldbaum M, Cai W et al (2018) Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 172:1122–1131 e1129
Cho YH, Lee SM, Seo JB et al (2018) Quantitative assessment of pulmonary vascular alterations in chronic obstructive lung disease: associations with pulmonary function test and survival in the KOLD cohort. Eur J Radiol 108:276–282
Lynch DA, Moore CM, Wilson C et al (2018) CT-based visual classification of emphysema: association with mortality in the COPDGene study. Radiology 288:859–866
Peng L, Lin L, Hu H et al (2019) Classification and quantification of emphysema using a multi-scale residual network. IEEE J Biomed Health Inform 23:2526–2536
Nambu A, Zach J, Schroeder J et al (2016) Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur J Radiol 85:2144–2151
Gonzalez G, Ash SY, Vegas-Sanchez-Ferrero G et al (2018) Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med 197:193–203
Hatt CR, Galban CJ, Labaki W, Kazerooni EA, Han ML (2018) Convolutional neural network based COPD and emphysema classifications are predictive of lung cancer diagnosis. Image Analysis for Moving Organ, Breast, and Thoracic Images:11040
Tang LYW, Coxson HO, Lam S, Leipsic J, Tam RC, Sin DD (2020) Towards large-scale case-finding: training and validation of residual networks for detection of chronic obstructive pulmonary disease using low-dose CT. Lancet Digit Health 2:e259–e267
National Lung Screening Trial Research T, Aberle DR, Adams AM et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Xu C, Qi S, Feng J et al (2020) DCT-MIL: deep CNN transferred multiple instance learning for COPD identification using CT images. Phys Med Biol 65:145011
Yan X, Tao M, Feng Q, Zhong P, Chang IC (2014) Deep learning of feature representation with multiple instance learning for medical image analysis. IEEE International Conference on Acoustics,
Shen Y, Wu N, Phang J et al (2021) An interpretable classifier for high-resolution breast cancer screening images utilizing weakly supervised localization. Med Image Anal 68:101908
Ilse M, Tomczak JM, Welling M (2018) Attention-based deep multiple instance learning. The 35th International Conference on Machine Learning,
Alom MZ, Yakopcic C, Hasan M, Taha TM, Asari VK (2019) Recurrent residual U-Net for medical image segmentation. J Med Imaging (Bellingham) 6:014006
Fluss R, Faraggi D, Reiser B (2005) Estimation of the Youden Index and its associated cutoff point. Biom J 47:458–472
Young KA, Strand M, Ragland MF et al (2019) Pulmonary subtypes exhibit differential global initiative for chronic obstructive lung disease spirometry stage progression: the COPDGene(R) study. Chronic Obstr Pulm Dis 6:414–429
Kinney GL, Santorico SA, Young KA et al (2018) Identification of chronic obstructive pulmonary disease axes that predict all-cause mortality: the COPDGene study. Am J Epidemiol 187:2109–2116
Woodruff PG, Barr RG, Bleecker E et al (2016) Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 374:1811–1821
Lowe KE, Regan EA, Anzueto A et al (2019) COPDGene((R)) 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis 6:384–399
Esteva A, Robicquet A, Ramsundar B et al (2019) A guide to deep learning in healthcare. Nat Med 25:24–29
Pino Pena I, Cheplygina V, Paschaloudi S et al (2018) Automatic emphysema detection using weakly labeled HRCT lung images. PLoS One 13:e0205397
Acknowledgements
We thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review.
Funding
This work was supported by the National Key R&D Program (2018YFC1313700), the National Natural Science Foundation of China (grant nos. 82100089, 81870064, and 82070086), and the “Gaoyuan” project of Pudong Health and Family Planning Commission (PWYgy2018-06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is professor Qiang Li, the head of the Department of Pulmonary and Critical Care Medicine of Shanghai East Hospital, Tongji University School of Medicine.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Informed consent
The requirement for written informed consent was waived due to the retrospective nature of the study.
Ethical approval
This study was approved by the ethics commissions of all participating hospitals, including the Affiliated Hospital of Qingdao University, Changsha First Hospital, People’s Liberation Army Joint Logistic Support Force 920th Hospital, and Shandong Provincial Hospital.
Methodology
• retrospective
• diagnostic or prognostic study
• multi-center study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: in this article only the authors Jiaxing Sun and Yusheng Yan were initially listed as having equally contributed to this work. This has been corrected to include the author Ximing Liao, as all three have contributed equally to the data collection, analyzing and drafting of the manuscript.
Supplementary Information
ESM 1
(DOCX 5694 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Liao, X., Yan, Y. et al. Detection and staging of chronic obstructive pulmonary disease using a computed tomography–based weakly supervised deep learning approach. Eur Radiol 32, 5319–5329 (2022). https://doi.org/10.1007/s00330-022-08632-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08632-7