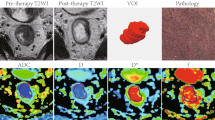
Abstract
Objectives
To examine the usefulness of the texture analysis (TA) of apparent diffusion coefficient (ADC) maps in predicting the chemoradiotherapy (CRT) response of muscle-invasive bladder cancer (MIBC).
Methods
We reviewed 45 MIBC patients who underwent cystectomy after CRT. CRT response was assessed through histologic evaluation of cystectomy specimens. Two radiologists determined the volume of interest for the index lesions on ADC maps of pretherapeutic 1.5-T MRI and performed TA using the LIFEx software. Forty-six texture features (TFs) were selected based on their contribution to the prediction of CRT sensitivity. To evaluate diagnostic performance, diagnostic models from the selected TFs were created using random forest (RF) and support vector machine (SVM), respectively.
Results
Twenty-three patients achieved pathologic complete response (pCR) to CRT. The feature selection identified first quartile ADC (Q1 ADC), gray-level co-occurrence matrix (GLCM) correlation, and GLCM homogeneity as important in predicting CRT response. Patients who achieved pCR showed significantly lower Q1 ADC and GLCM correlation values (0.66 × 10−3 mm2/s and 0.53, respectively) than those who did not (0.81 × 10−3 mm2/s and 0.70, respectively; p < 0.05 for both). The AUCs of the RF and SVM models incorporating the selected TFs were 0.82 (95% confidence interval [CI]: 0.67–0.97) and 0.96 (95% CI: 0.91–1.00), respectively, and the AUC of the SVM model was better than that of the mean ADC value (0.76, 95% CI: 0.61–0.90; p = 0.0037).
Conclusion
TFs can serve as imaging biomarkers in MIBC patients for predicting CRT sensitivity. TAs of ADC maps can potentially optimize patient selection for CRT.
Key Points
• Texture analysis of ADC maps and feature selection identified important texture features for classifying pathologic tumor response in patients with muscle-invasive bladder cancer.
• The machine learning model incorporating the texture features set, which included first quartile ADC, GLCM correlation, and GLCM homogeneity, showed high performance in predicting chemoradiotherapy response.
• Texture features could serve as imaging biomarkers that optimize eligible patient selection for chemoradiotherapy in muscle-invasive bladder cancer.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CR:
-
Complete response
- CRT:
-
Chemoradiotherapy
- DWI:
-
Diffusion-weighted imaging
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLZLM:
-
Gray-level zone length matrix
- IQR:
-
Interquartile range
- MIBC:
-
Muscle-invasive bladder cancer
- MRI:
-
Magnetic resonance imaging
- NGLDM:
-
Neighborhood gray-level different matrix
- pCR:
-
Pathologic CR
- Q1:
-
First quartile
- Q2:
-
Second quartile
- RF:
-
Random forest
- ROC:
-
Receiver operating characteristic
- ROIs:
-
Regions of interest
- SRE:
-
Short run emphasis
- SVM:
-
Support vector machine
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- TA:
-
Texture analysis
- TF:
-
Texture feature
- TUR:
-
Transurethral resection
- VOI:
-
Volume of interest
- ZE:
-
Zone percentage
References
Chang SS, Bochner BH, Chou R et al (2017) Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 198:552–559
Mitin T, George A, Zietman AL et al (2016) Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: a pooled analysis of NRG oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys 94:67–74
Ploussard G, Daneshmand S, Efstathiou JA et al (2014) Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol 66:120–137
Panebianco V, Narumi Y, Altun E et al (2018) Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (vesical imaging-reporting and data system). Eur Urol 74:294–306
Expert Panel on Urologic Imaging, Allen BC, Oto A et al (2019) ACR appropriateness criteria(R) post-treatment surveillance of bladder cancer. J Am Coll Radiol 16:S417–S427
Zhu HB, Zhang XY, Zhou XH et al (2017) Assessment of pathological complete response to preoperative chemoradiotherapy by means of multiple mathematical models of diffusion-weighted MRI in locally advanced rectal cancer: a prospective single-center study. J Magn Reson Imaging 46:175–183
Jones M, Hruby G, Stanwell P et al (2015) Multiparametric MRI as an outcome predictor for anal canal cancer managed with chemoradiotherapy. BMC Cancer 15:281
Wang L, Liu L, Han C et al (2016) The diffusion-weighted magnetic resonance imaging (DWI) predicts the early response of esophageal squamous cell carcinoma to concurrent chemoradiotherapy. Radiother Oncol 121:246–251
Yoshida S, Koga F, Kobayashi S et al (2012) Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 83:e21–e27
Ji GW, Zhang YD, Zhang H et al (2019) Biliary tract cancer at CT: a radiomics-based model to predict lymph node metastasis and survival outcomes. Radiology 290:90–98
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Gu D, Hu Y, Ding H et al (2019) CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol. https://doi.org/10.1007/s00330-019-06176-x
Limkin EJ, Sun R, Dercle L et al (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol 28:1191–1206
Shi Z, Yang Z, Zhang G et al (2013) Characterization of texture features of bladder carcinoma and the bladder wall on MRI: initial experience. Acad Radiol 20:930–938
Zhang X, Xu X, Tian Q et al (2017) Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J Magn Reson Imaging 46:1281–1288
Wang H, Hu D, Yao H et al (2019) Radiomics analysis of multiparametric MRI for the preoperative evaluation of pathological grade in bladder cancer tumors. Eur Radiol 29:6182–6190
Xu X, Zhang X, Tian Q et al (2019) Quantitative identification of nonmuscle-invasive and muscle-invasive bladder carcinomas: a multiparametric MRI radiomics analysis. J Magn Reson Imaging 49:1489–1498
Xu S, Yao Q, Liu G et al (2020) Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur Radiol 30:1804–1812
Wang H, Xu X, Zhang X et al (2020) Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: a double-center study. Eur Radiol 30:4816–4827
Koga F, Yoshida S, Kawakami S et al (2008) Low-dose chemoradiotherapy followed by partial or radical cystectomy against muscle-invasive bladder cancer: an intent-to-treat survival analysis. Urology 72:384–388
Koga F, Kihara K, Fujii Y et al (2009) Favourable outcomes of patients with clinical stage T3N0M0 bladder cancer treated with induction low-dose chemo-radiotherapy plus partial or radical cystectomy vs immediate radical cystectomy: a single-institutional retrospective comparative study. BJU Int 104:189–194
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789
Kursa MB, Rudnicki WR (2010) Feature selection with the Boruta package. J Stat Softw 36:1–13
Breiman L (2001) Random forests. Mach Learn 45:5–32
Noble WS (2006) What is a support vector machine? Nat Biotechnol 24:1565–1567
Kobayashi S, Koga F, Yoshida S et al (2011) Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 21:2178–2186
Yoshida S, Takahara T, Kwee TC, Waseda Y, Kobayashi S, Fujii Y (2017) DWI as an imaging biomarker for bladder cancer. AJR Am J Roentgenol 208:1218–1228
de Haas RJ, Steyvers MJ, Futterer JJ (2014) Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol 202:1187–1195
Tuncbilek N, Kaplan M, Altaner S et al (2009) Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. AJR Am J Roentgenol 192:949–955
Tanaka H, Kijima T, Fujii Y (2020) Bladder preservation therapy in muscle-invasive bladder cancer: current evidence and future perspectives. AME Med J 5:16–16
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yasuhisa Fujii.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study cohorts have been previously reported in International Journal of Radiation Oncology, Biology, Physics (https://doi.org/10.1016/j.ijrobp.2011.11.065).
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, K., Yoshida, S., Tsuchiya, J. et al. Usefulness of texture features of apparent diffusion coefficient maps in predicting chemoradiotherapy response in muscle-invasive bladder cancer. Eur Radiol 32, 671–679 (2022). https://doi.org/10.1007/s00330-021-08110-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08110-6