Abstract
Objectives
The goal of this study was to assess the relationship between liver surface nodularity (LSN), chemotherapy-associated liver injury (CALI), and clinically relevant post-hepatectomy liver failure (CR-PHLF) (i.e., ≥ grade B) in patients undergoing hepatectomy for colorectal liver metastases (CLM).
Methods
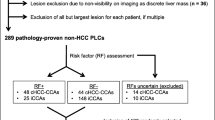
Preoperative CT scans of patients who underwent chemotherapy followed by hepatectomy for CLM between 2010 and 2017 were retrospectively analyzed. LSN was measured using semi-automated CT software CT images in patients who had available preoperative CT scans within 6 weeks before hepatectomy, and was computed based on the means of one to 10 measurements by two abdominal radiologists consensually. The association of LSN, CALI, and CR-PHLF was analyzed.
Results
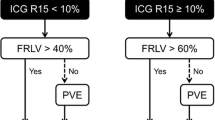
Two hundred fifty-six patients were analyzed (149 men and 107 women; overall median age, 61 [range, 29–88 years]). A total of 26 patients (10.2%) developed CR-PHLF. The optimal LSN cut-off value for detecting CR-PHLF was 2.5, as determined by receiver operative characteristic analysis (p < 0.001). LSN ≥ 2.5 was associated with prolonged chemotherapy (> 6 cycles, p = 0.018), but not with CALIs. After propensity score matching, LSN remained significantly associated with CR-PHLF (p = 0.031). Furthermore, multivariate analysis identified LSN ≥ 2.50 and future liver remnant (FLR) < 30% as significant preoperative predictors of CR-PHLF in 102 patients undergoing major hepatectomy. LSN ≥ 2.50 was more frequent in patients undergoing major hepatectomy despite FLR ≥ 30% (p = 0.008).
Conclusion
LSN quantified on CT is an independent surrogate of CR-PHLF in patients who undergo chemotherapy followed by hepatectomy for CLM and may provide a valuable additional tool in the preoperative assessment of these patients.
Key Points
• LSN was not associated with chemotherapy- associated liver injury but high LSN (defined ≥ 2.5) was associated with prolonged chemotherapy (> 6 cycles).
• High LSN was an independent predictor of clinically relevant postoperative liver failure in patients undergoing hepatectomy for CRLM.
• LSN ≥ 2.50 was more frequent in patients with PHLF after major hepatectomy despite a future liver remnant ≥ 30%.



Similar content being viewed by others
Abbreviations
- (CR)-PHLF:
-
(Clinically relevant) post-hepatectomy liver failure
- ALPPS:
-
Associating liver partition and portal vein ligation for staged hepatectomy
- APRI:
-
Aspartate amino transferase-to-platelet ratio index
- CALI:
-
Chemotherapy-associated liver injury
- CASH:
-
Chemotherapy-associated steatohepatitis
- CLM:
-
Colorectal liver metastases
- FIB-4:
-
Fibrosis-4
- FLR:
-
Future liver remnant
- LSN:
-
Liver surface nodularity
- NRH:
-
Nodular regenerative hyperplasia
- SOS:
-
Sinusoidal obstruction syndrome
References
Vigano L, De Rosa G, Toso C et al (2017) Reversibility of chemotherapy-related liver injury. J Hepatol 67:84–91
Adam R, De Gramont A, Figueras J et al (2012) The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 17:1225–1239
Soubrane O, Brouquet A, Zalinski S et al (2010) Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg 251:454–460
Dorcaratto D, Mazzinari G, Fernandez M et al (2019) Impact of postoperative complications on survival and recurrence after resection of colorectal liver metastases: systematic review and meta-analysis. Ann Surg 270(6):1018–1027
Farid SG, Aldouri A, Morris-Stiff G et al (2010) Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg 251:91–100
Araujo RL, Gönen M, Herman P (2015) Chemotherapy for patients with colorectal liver metastases who underwent curative resection improves long-term outcomes: systematic review and meta-analysis. Ann Surg Oncol 22:3070–3078
Rahbari NN, Reissfelder C, Koch M et al (2011) The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 18:3640–3649
Rubbia-Brandt L, Audard V, Sartoretti P et al (2004) Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 15:460–466
Kneuertz PJ, Maithel SK, Staley CA, Kooby DA (2011) Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol 18:181–190
Smith AD, Branch CR, Zand K et al (2016) Liver surface nodularity quantification from routine ct images as a biomarker for detection and evaluation of cirrhosis. Radiology 280:771–781
Sartoris R, Rautou PE, Elkrief L et al (2018) Quantification of liver surface nodularity at ct: utility for detection of portal hypertension. Radiology 289:698–707
Catania R, Furlan A, Smith AD, Behari J, Tublin M, Borhani A (2020) Diagnostic value of MRI-derived liver surface nodularity score for the non-invasive quantification of hepatic fibrosis in non-alcoholic fatty liver disease. Eur Radiol. https://doi.org/10.1007/s00330-020-07114-y
Rahbari NN, Garden OJ, Padbury R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Sartoris R, Lazareth M, Nivolli A, Dioguardi Burgio M, Vilgrain V, Ronot M (2020) CT-based liver surface nodularity for the detection of clinically significant portal hypertension: defining measurement quality criteria. Abdom Radiol (NY). https://doi.org/10.1007/s00261-020-02519-1
Bedossa P, Poitou C, Veyrie N et al (2012) Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56:1751–1759
Wanless IR (1990) Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 11:787–797
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J Hepatobiliary PancreatSurg 12:351–355
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 40:205–213
Friedman SF, Martin P, Munoz JS (2003) Laboratory evaluation of the patient with liver disease. Hepatology, a textbook of liver disease. Philedelphia; Saunders publication 1:661–709
Pesce A, Scilletta R, Branca A et al (2012) Does transient elastography (FibroScan®) have a role in decision making in hepatocellularcarcinoma? HPB (Oxford) 14:403–408
Overman MJ, Maru DM, Charnsangavej C et al (2010) Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 28:2549–2555
Rubbia-Brandt L, Lauwers GY, Wang H et al (2010) Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology 56:430–439
Hobeika C, Cauchy F, Sartoris R et al (2020) Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br J Surg. https://doi.org/10.1002/bjs.11511
Nakano H, Oussoultzoglou E, Rosso E et al (2008) Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 247:118–124
Vauthey JN, Mizuno T, Overman MJ, Soubrane O (2017) Can we navigate chemotherapy-induced hepatic injuries from pathology to bedside? J Hepatol 67:10–11
Yamashita S, Shindoh J, Mizuno T et al (2017) Hepatic atrophy following preoperative chemotherapy predicts hepatic insufficiency after resection of colorectal liver metastases. J Hepatol 67:56–64
Niekamp AS, Huang SY, Mahvash A et al (2020) Hepatic vein embolization after portal vein embolization to induce additional liver hypertrophy in patients with metastatic colorectal carcinoma. Eur Radiol 30:3862–3868
Shindoh J, Tzeng CW, Aloia TA et al (2013) Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol 20:2493–2500
Narita M, Oussoultzoglou E, Fuchshuber P et al (2012) What is a safe future liver remnant size in patients undergoing major hepatectomy for colorectal liver metastases and treated by intensive preoperative chemotherapy? Ann Surg Oncol 19(8):2526–2538
De Vos N, Sartoris R, Cauchy F, Rautou PE, Vilgrain V, Ronot M (2020) Performance of liver surface nodularity quantification for the diagnosis of portal hypertension in patients with cirrhosis: comparison between MRI with hepatobiliary phase sequences and CT. Abdom Radiol (NY) 45(2):365–372
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maxime Ronot.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2769 kb)
Rights and permissions
About this article
Cite this article
Yoh, T., Perrot, A., Beaufrère, A. et al. Liver surface nodularity: a novel predictor of post-hepatectomy liver failure in patients with colorectal liver metastases following chemotherapy. Eur Radiol 31, 5830–5839 (2021). https://doi.org/10.1007/s00330-020-07683-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07683-y