Abstract
Objectives
To evaluate a deep learning–based model using model-generated segmentation masks to differentiate invasive pulmonary adenocarcinoma (IPA) from preinvasive lesions or minimally invasive adenocarcinoma (MIA) on CT, making comparisons with radiologist-derived measurements of solid portion size.
Methods
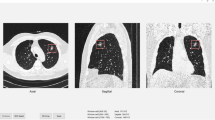
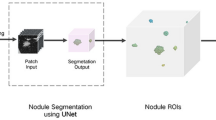
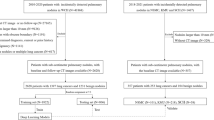
Four hundred eleven subsolid nodules (SSNs) (120 preinvasive lesions or MIAs and 291 IPAs) in 333 patients who underwent surgery between June 2010 and August 2016 were retrospectively included to develop the model (370 SSNs in 293 patients for training and 41 SSNs in 40 patients for tuning). Ninety SSNs of 2 cm or smaller (45 preinvasive lesions or MIAs and 45 IPAs) resected in 2018 formed a validation set. Six radiologists measured the solid portion of each nodule. Performances of the model and radiologists were assessed using receiver operating characteristics curve analysis.
Results
The deep learning model differentiated IPA from preinvasive lesions or MIA with areas under the curve (AUCs) of 0.914, 0.956, and 0.833 for the training, tuning, and validation sets, respectively. The mean AUC of the radiologists was 0.835 in the validation set, without significant differences between radiologists and the model (p = 0.97). The sensitivity, specificity, and accuracy of the model were 71% (32/45), 87% (39/45), and 79% (71/90), respectively, whereas the corresponding values of the radiologists were 75.2% (203/270), 76.7% (207/270), and 75.9% (410/540) with a 5-mm threshold for the solid portion size.
Conclusions
The performance of the model for differentiating IPA from preinvasive lesions or MIA was comparable to that of the radiologists’ measurements of solid portion size.
Key Points
• A deep learning–based model differentiated IPA from preinvasive lesions or MIA with AUCs of 0.914 and 0.956 for the training and tuning sets, respectively.
• In the validation set including subsolid nodules of 2 cm or smaller, the model showed an AUC of 0.833, being on par with the performance of the solid portion size measurements made by the radiologists (AUC, 0.835; p = 0.97).
• SSNs with a solid portion measuring > 10 mm on CT showed a high probability of being IPA (positive predictive value, 93.5–100.0%).




Similar content being viewed by others
Abbreviations
- AAH:
-
Atypical adenomatous hyperplasia
- AIS:
-
Adenocarcinoma in situ
- AUROC:
-
Area under the receiver-operating characteristics curve
- CI:
-
Confidence interval
- CNN:
-
Convolutional neural network
- ICC:
-
Intra-class correlation coefficient
- IPA:
-
Invasive pulmonary adenocarcinoma
- MIA:
-
Minimally invasive adenocarcinoma
- SSN:
-
Subsolid nodule
References
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284:228–243
Cho J, Ko SJ, Kim SJ et al (2014) Surgical resection of nodular ground-glass opacities without percutaneous needle aspiration or biopsy. BMC Cancer 14:838
Lee SM, Park CM, Song YS et al (2017) CT assessment-based direct surgical resection of part-solid nodules with solid component larger than 5 mm without preoperative biopsy: experience at a single tertiary hospital. Eur Radiol 27:5119–5126
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J (2008) Fleischner society: glossary of terms for thoracic imaging. Radiology 246:697–722
Henschke CI, Yankelevitz DF, Mirtcheva R et al (2002) CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 178:1053–1057
Hattori A, Hirayama S, Matsunaga T et al (2019) Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adenocarcinoma. J Thorac Oncol 14:265–275
Tsutani Y, Miyata Y, Nakayama H et al (2014) Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 145:66–71
Jin C, Cao J, Cai Y et al (2017) A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg 153:462–469 e461
Yankelevitz DF, Yip R, Smith JP et al (2015) CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology 277:555–564
Lee JH, Park CM, Kim H, Hwang EJ, Park J, Goo JM (2017) Persistent part-solid nodules with solid part of 5 mm or smaller: can the ‘follow-up and surgical resection after interval growth’ policy have a negative effect on patient prognosis? Eur Radiol 27:195–202
Zhang Y, Shen Y, Qiang JW, Ye JD, Zhang J, Zhao RY (2016) HRCT features distinguishing pre-invasive from invasive pulmonary adenocarcinomas appearing as ground-glass nodules. Eur Radiol 26:2921–2928
Kim H, Goo JM, Park CM (2019) A simple prediction model using size measures for discrimination of invasive adenocarcinomas among incidental pulmonary subsolid nodules considered for resection. Eur Radiol 29:1674–1683
Son JY, Lee HY, Lee KS et al (2014) Quantitative CT analysis of pulmonary ground-glass opacity nodules for the distinction of invasive adenocarcinoma from pre-invasive or minimally invasive adenocarcinoma. PLoS One 9:e104066
Fan L, Fang M, Li Z et al (2019) Radiomics signature: a biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur Radiol 29:889–897
Zhao W, Yang J, Sun Y et al (2018) 3D deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res 78:6881–6889
Kim H, Lee D, Cho WS et al (2020) CT-based deep learning model to differentiate invasive pulmonary adenocarcinomas appearing as subsolid nodules among surgical candidates: comparison of the diagnostic performance with a size-based logistic model and radiologists. Eur Radiol. https://doi.org/10.1007/s00330-019-06628-4
Cohen JG, Reymond E, Lederlin M et al (2015) Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 84:738–744
Travis WD, Brambilla E, Noguchi M et al (2011) International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285
Yue X, Liu S, Liu S et al (2018) HRCT morphological characteristics distinguishing minimally invasive pulmonary adenocarcinoma from invasive pulmonary adenocarcinoma appearing as subsolid nodules with a diameter of </=3 cm. Clin Radiol 73:411 e417–411 e415
Rami-Porta R, Bolejack V, Crowley J et al (2015) The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung Cancer. J Thorac Oncol 10:990–1003
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Wang S, Zhou M, Liu Z et al (2017) Central focused convolutional neural networks: developing a data-driven model for lung nodule segmentation. Med Image Anal 40:172–183
Lee KH, Goo JM, Park SJ et al (2014) Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 9:74–82
Ko JP, Suh J, Ibidapo O et al (2016) Lung adenocarcinoma: correlation of quantitative CT findings with pathologic findings. Radiology 280:931–939
Funding
This study received funding from the Industrial Strategic technology development program (10072064, Development of Novel Artificial Intelligence Technologies To Assist Imaging Diagnosis of Pulmonary, Hepatic, and Cardiac Diseases and Their Integration into Commercial Clinical PACS Platforms) funded by the Ministry of Trade Industry and Energy (MI, Korea).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sang Min Lee.
Conflict of interest
Three of the authors of this manuscript (Gwangbeen Park, Hyunho Park, and Kyuhwan Jung) belong to VUNO Inc., Seoul, South Korea.
Statistics and biometry
Seonok Kim, who is a statistician in Asan medical center, provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical Approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 379 kb)
Rights and permissions
About this article
Cite this article
Park, S., Park, G., Lee, S.M. et al. Deep learning–based differentiation of invasive adenocarcinomas from preinvasive or minimally invasive lesions among pulmonary subsolid nodules. Eur Radiol 31, 6239–6247 (2021). https://doi.org/10.1007/s00330-020-07620-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07620-z