Abstract
Objectives
The LI-RADS M (LR-M) category describes hepatic lesions probably or definitely malignant, but not specific for hepatocellular carcinoma in at-risk patients. Differentiation among LR-M entities, particularly detecting cholangiocarcinoma-containing tumors (M-CCs), is essential for treatment and prognosis. Thus, we aimed to develop diagnostic models on gadoxetate disodium–enhanced MRI comprising serum tumor markers and LI-RADS imaging features for M-CC.
Methods
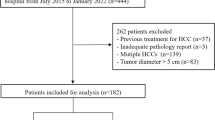
Consecutive at-risk patients with LR-M lesions exclusively (no co-existing LR-4 and/or LR-5 lesions) were retrieved retrospectively from a prospectively collected database spanning 3 years. Intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular-cholangiocarcinoma (c-HCC-CCA) were classified together as M-CC. LI-RADS features determined by three independent radiologists and clinically relevant serum tumor markers were used to generate M-CC diagnostic models through logistic regression analysis against histology. Per-patient performance was evaluated using area under the receiver operating curve (AUC), sensitivity, and specificity.
Results
Forty-five patients were included, 42.2% (19/45) with hepatocellular carcinoma, 33.3% (15/45) with ICC, 13.3% (6/45) with c-HCC-CCA, and 11.1% (5/45) with other hepatic lesions. Carbohydrate antigen (CA)19-9 > 38 U/mL, α-fetoprotein (AFP) > 4.8 ng/mL, and absence of the LI-RADS feature “blood products in mass” were significant predictors of M-CC. Combining three predictors demonstrated AUC of 0.862, sensitivity of 76%, and specificity of 88%. The risk of M-CC with all three criteria fulfilled was 98% (AUC, 0.690; sensitivity, 38%; specificity, 100%).
Conclusions
In at-risk patients with LR-M lesions, integrating CA19-9, AFP, and the LI-RADS feature “blood products in mass” achieved high diagnostic performance for M-CC. When all three criteria were fulfilled, the specificity for M-CC was 100%.
Key Points
• In at-risk patients who had LR-M lesions exclusively (no concomitant LR-4/5 lesions), a model with carbohydrate antigen > 38 U/mL, α-fetoprotein > 4.8 ng/mL, and absence of the LI-RADS feature “blood products in mass” achieved high accuracy for diagnosing cholangiocarcinoma-containing tumors.
• In patients of whom all three criteria were fulfilled, the specificity for M-CC was 100%, which might reduce or eliminate the need for biopsy confirmation.




Similar content being viewed by others
Abbreviations
- AFP:
-
α-Fetoprotein
- CA:
-
Carbohydrate antigen
- c-HCC-CCA:
-
Combined hepatocellular-cholangiocarcinoma
- EOB-MRI:
-
Gadoxetate disodium–enhanced magnetic resonance imaging
- ICC:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- M-CC:
-
Cholangiocarcinoma-containing tumors
References
American College of Radiology (2018) CT/MRI LI-RADS version 2018. Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed November 1, 2019
Kierans AS, Makkar J, Guniganti P et al (2019) Validation of Liver Imaging Reporting and Data System 2017 (LI-RADS) criteria for imaging diagnosis of hepatocellular carcinoma. J Magn Reson Imaging 49(7):e205–e215
Lee SM, Lee JM, Ahn SJ et al (2019) LI-RADS version 2017 versus version 2018: diagnosis of hepatocellular carcinoma on gadoxetate disodium-enhanced MRI. Radiology 292(3):655–663
van der Pol CB, Lim CS, Sirlin CB et al (2019) Accuracy of the Liver Imaging Reporting and Data System in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology 156(4):976–986
Tang A, Bashir MR, Corwin MT et al (2018) Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 286(1):29–48
Yin X, Zhang BH, Qiu SJ et al (2012) Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol 19(9):2869–2876
Brunt E, Aishima S, Clavien PA et al (2018) cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 68(1):113–126
Sapisochin G, Fidelman N, Roberts JP, Yao FY (2011) Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 17(8):934–942
Bridgewater J, Galle PR, Khan SA et al (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289
Joo I, Lee JM, Yoon JH (2018) Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology 288(1):7–13
Clements O, Eliahoo J, Kim JU et al (2020) Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol 72(1):95–103
Kim JH, Joo I, Lee JM (2019) Atypical appearance of hepatocellular carcinoma and its mimickers: how to solve challenging cases using gadoxetic acid-enhanced liver magnetic resonance imaging. Korean J Radiol 20(7):1019–1041
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723–750
Kim YY, Kim MJ, Kim EH et al (2019) Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS Version 2018. Radiology 291(1):72–80
Choi SH, Lee SS, Park SH et al (2019) LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology 290(2):388–397
Fraum TJ, Tsai R, Rohe E et al (2018) Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver Imaging Reporting and Data System Version 2014. Radiology 286(1):158–172
Choi SH, Lee SS, Kim SY et al (2017) Intrahepatic cholangiocarcinoma in patients with cirrhosis: differentiation from hepatocellular carcinoma by using gadoxetic acid-enhanced MR imaging and dynamic CT. Radiology 282(3):771–781
Park HJ, Jang KM, Kang TW et al (2016) Identification of imaging predictors discriminating different primary liver tumours in patients with chronic liver disease on gadoxetic acid-enhanced MRI: a classification tree analysis. Eur Radiol 26(9):3102–3111
Ludwig DR, Fraum TJ, Cannella R et al (2019) Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol (NY) 44(6):2116–2132
Horvat N, Nikolovski I, Long N et al (2018) Imaging features of hepatocellular carcinoma compared to intrahepatic cholangiocarcinoma and combined tumor on MRI using liver imaging and data system (LI-RADS) version 2014. Abdom Radiol (NY) 43(1):169–178
Hwang J, Kim YK, Min JH et al (2017) Capsule, septum, and T2 hyperintense foci for differentiation between large hepatocellular carcinoma (≥5 cm) and intrahepatic cholangiocarcinoma on gadoxetic acid MRI. Eur Radiol 27(11):4581–4590
Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD (2008) Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 48(4):1106–1117
Wang M, Gao Y, Feng H et al (2018) A nomogram incorporating six easily obtained parameters to discriminate intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Cancer Med 7(3):646–654
Chinese Society of Hepatology, Chinese Medical Association (2019) Chinese guidelines on the management of liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi 27(11):846–865
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. World Health Organization, Geneva
Cerny M, Chernyak V, Olivié D et al (2018) LI-RADS version 2018 ancillary features at MRI. Radiographics 38(7):1973–2001
Cerny M, Bergeron C, Billiard JS et al (2018) LI-RADS for MR imaging diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Radiology 288(1):118–128
Liang B, Zhong L, He Q et al (2015) Diagnostic accuracy of serum CA19-9 in patients with cholangiocarcinoma: a systematic review and meta-analysis. Med Sci Monit 21:3555–3563
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11(4):317–370
Marrero JA, Feng Z, Wang Y et al (2009) Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 137(1):110–118
Lee HS, Kim MJ, An C (2019) How to utilize LR-M features of the LI-RADS to improve the diagnosis of combined hepatocellular-cholangiocarcinoma on gadoxetate-enhanced MRI? Eur Radiol 29(5):2408–2416
Elsayes KM, Fowler KJ, Chernyak V et al (2019) User and system pitfalls in liver imaging with LI-RADS. J Magn Reson Imaging 50(6):1673–1686
Kim YY, Choi JY, Sirlin CB et al (2019) Pitfalls and problems to be solved in the diagnostic CT/MRI Liver Imaging Reporting and Data System (LI-RADS). Eur Radiol 29(3):1124–1132
Funding
This study has received funding by Research Grant of National Natural Science Foundation of China (No. 81771797).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hanyu Jiang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors, Dong Xiao and Alaattin Erkanli, have significant statistical expertise.
Informed consent
Written informed consent was not required for this study because we retrospectively analyzed data from a prospectively collected cohort (Clinical trial registration No. ChiCTR1900026668).
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
In a previous study (Jiang H, Liu X, Chen J, et al (2019) Man or machine? Prospective comparison of the version 2018 EASL, LI-RADS criteria and a radiomics model to diagnose hepatocellular carcinoma. Cancer Imaging 19(1):84), we reported 30 patients included in the current study. While the previous work evaluated and compared the diagnostic accuracies of EASL v2018, LI-RADS v2018 criteria, and a radiomics model for HCC, the current study focused on the detection of M-CC in LR-M patients using a quite different methodology.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Jiang, H., Song, B., Qin, Y. et al. Diagnosis of LI-RADS M lesions on gadoxetate-enhanced MRI: identifying cholangiocarcinoma-containing tumor with serum markers and imaging features. Eur Radiol 31, 3638–3648 (2021). https://doi.org/10.1007/s00330-020-07488-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07488-z