Abstract
Objective
Assessment of lung development and maturity is of utmost importance in prenatal counseling. Blood oxygen level–dependent (BOLD) effect MRI was developed for functional evaluations of organs. To date, no data are available in fetal lungs and nothing is known about the existence of a BOLD effect in the lungs. The aim of our study was to evaluate if a BOLD response could be detected in fetal lungs.
Materials and methods
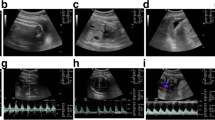
From January 2014 to December 2016, 38 healthy pregnant women were prospectively enrolled. After a routine scan on a 1.5-T MRI device (normoxic period), maternal hyperoxia was induced for 5 min before the BOLD sequence (hyperoxic period). R2* was evaluated by fitting average intensity of the signal, both for normoxic (norm) and hyperoxic (hyper) periods.
Results
A significant BOLD response was observed after maternal hyperoxia in the lungs with a mean R2* decrease of 12.1 ± 2.5% (p < 0.001), in line with the placenta response with a mean R2* decrease of 19.2 ± 5.9% (p < 0.0001), confirming appropriate oxygen uptake. Conversely, no significant BOLD effect was observed for the brain nor the liver with a mean ∆R2* of 3.6 ± 3.1% (p = 0.64) and 2.8 ± 3.7% (p = 0.23).
Conclusion
This study shows for the first time in human that a BOLD response can be observed in the normal fetal lung despite its prenatal “non-functional status.” If confirmed in congenital lung and chest malformations, this property could be used in addition to the lung volume for a better prediction of postnatal respiratory status.
Key Points
• Blood oxygen level–dependent (BOLD) effect MRI was developed for functional evaluations of organs and could have interesting implications for the fetal organs.
• Assessment of lung development is of utmost importance in prenatal counseling, but to date no data are available in fetal lungs.
• BOLD response can be observed in the normal fetal lung opening the way to studies on fetus with pathological lungs.




Similar content being viewed by others
Abbreviations
- BOLD:
-
Blood oxygen level–dependent
- GA:
-
Gestational age
- LHR:
-
Lung-to-head ratio
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Regions of interest
- SEM:
-
Standard error to the mean
- TE:
-
Echo time effective
- TR:
-
Repetition
References
Marshall J, Salemi JL, Tanner JP et al (2015) Prevalence, correlates, and outcomes of omphalocele in the United States, 1995-2005. Obstet Gynecol 126(2):284–293
Morris JK, Springett AL, Greenlees R et al (2018) Trends in congenital anomalies in Europe from 1980 to 2012. PLoS One 13(4):e0194986
Ramakrishnan R, Salemi JL, Stuart AL et al (2018) Trends, correlates, and survival of infants with congenital diaphragmatic hernia and its subtypes. Birth Defects Res 6
Donn SM, Sinha SK (2017) Pulmonary diagnostics. Semin Fetal Neonatal Med 22(4):200–205
Quinn TM, Hubbard AM, Adzick NS (1998) Prenatal magnetic resonance imaging enhances fetal diagnosis. J Pediatr Surg 33(4):553–558
Debus A, Hagelstein C, Kilian AK et al (2013) Fetal lung volume in congenital diaphragmatic hernia: association of prenatal MR imaging findings with postnatal chronic lung disease. Radiology. 266(3):887–895
Jani J, Cannie M, Sonigo P et al (2008) Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol 32:793–799
Shieh HF, Barnewolt CE, Wilson JM et al (2017) Percent predicted lung volume changes on fetal magnetic resonance imaging throughout gestation in congenital diaphragmatic hernia. J Pediatr Surg 52(6):933–937
Vergani P (2012) Prenatal diagnosis of pulmonary hypoplasia. Curr Opin Obstet Gynecol 24(2):89–94
Rubesova E (2016) Why do we need more data on MR volumetric measurements of the fetal lung? Pediatr Radiol 46(2):167–171
Russo FM, Eastwood MP, Keijzer R et al (2017) Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 49:704–713
Kasprian G, Balassy C, Brugger PC, Prayer D (2006) MRI of normal and pathological fetal lung development. Eur J Radiol 57(2):261–270
Cannie M, Jani J, De Keyzer F, Roebben I, Breysem L, Deprest J (2011) T2 quantifications of fetal lungs at MRI-normal ranges. Prenat Diagn 31(7):705–711
Terui K, Omoto A, Osada H et al (2011) Prediction of postnatal outcomes in congenital diaphragmatic hernia using MRI signal intensity of the fetal lung. J Perinatol 31(4):269–273
Moshiri M, Mannelli L, Richardson ML, Bhargava P, Dubinsky TJ (2013) Fetal lung maturity assessment with MRI fetal lung-to-liver signal-intensity ratio. AJR Am J Roentgenol 201(6):1386–1390
Lee W, Krisko A, Shetty A et al (2009) Non-invasive fetal lung assessment using diffusion-weighted imaging. Ultrasound Obstet Gynecol 34(6):673–677
Yablonskiy DA, Sukstanskii AL, He X (2013) Blood oxygenation level-dependent (BOLD)-based techniques for the quantification of brain hemodynamic and metabolic properties - theoretical models and experimental approaches. NMR Biomed 26:963–986
Cao J, Lu KH, Oleson ST et al (2019) Gastric stimulation drives fast BOLD responses of neural origin. Neuroimage. 15(197):200–211
Niles DJ, Artz NS, Djamali A, Sadowski EA, Grist TM, Fain SB (2016) Longitudinal assessment of renal perfusion and oxygenation in transplant donor-recipient pairs using arterial spin labeling and blood oxygen level-dependent magnetic resonance imaging. Invest Radiol 51:113–120
Grover S, Lloyd R, Perry R et al (2019) Assessment of myocardial oxygenation, strain, and diastology in MYBPC3-related hypertrophic cardiomyopathy: a cardiovascular magnetic resonance and echocardiography study. Eur Heart J Cardiovasc Imaging 20:932–938
Lee J, Kim CK, Gu KW, Park W (2019) Value of blood oxygenation level-dependent MRI for predicting clinical outcomes in uterine cervical cancer treated with concurrentchemoradiotherapy. Eur Radiol. 29(11):6256–6265
Siauve N, Chalouhi GE, Deloison B et al (2015) Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol 213(4 Suppl):S103–S114
Huen I, Morris DM, Wright C et al (2013) R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med 70(5):1427–1433
Semple SI, Wallis F, Haggarty P et al (2001) The measurement of fetal liver T(*)(2) in utero before and after maternal oxygen breathing: progress towards a non-invasive measurement of fetal oxygenation and placental function. Magn Reson Imaging 19(7):921–928
Sørensen A, Peters D, Simonsen C et al (2013) Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Prenat Diagn 33(2):141–145
Luo J, Abaci Turk E, Bibbo C et al (2017) In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep 7(1):3713
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1(8476):307–310
Chalouhi GE, Alison M, Deloison B et al (2013) Fetoplacental oxygenation in an intrauterine growth restriction rat model by using blood oxygen level-dependent MR imaging at 4.7 T. Radiology. 269(1):122–129
Ingram E, Morris D, Naish J, Myers J, Johnstone E (2017) MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology. 285(3):953–960
Kivilevitch Z, Salomon LJ, Yagel S, Achiron R (2011) Bowel circulation in normally grown and growth-restricted fetuses. J Ultrasound Med 30(11):1529–1537
Godfrey KM, Haugen G, Kiserud T et al (2012) Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PLoS One 7(8):e41759
Stampalija T, Casati D, Monasta L et al (2016) Brain sparing effect in growth-restricted fetuses is associated with decreased cardiac acceleration and deceleration capacities: a case-control study. BJOG. 123(12):1947–1954
Morton SU, Brodsky D (2016) Fetal physiology and the transition to extrauterine life. Clin Perinatol 43(3):395–407
Wu TW, Azhibekov T, Seri I (2016) Transitional hemodynamics in preterm neonates: clinical relevance. Pediatr Neonatol 57(1):7–18
DeKoninck P, Jimenez J, Russo FM, Hodges R, Gratacós E, Deprest J (2014) Assessment of pulmonary vascular reactivity to oxygen using fractional moving blood volume in fetuses with normal lung development and pulmonary hypoplasia in congenital diaphragmatic hernia. Prenat Diagn 34(10):977–981
Schoennagel BP, Yamamura J, Fischer R et al (2015) BOLD MRI in the brain of fetal sheep at 3T during experimental hypoxia. J Magn Reson Imaging 41:110–116
Sørensen A, Holm D, Pedersen M et al (2011) Left-right difference in fetal liver oxygenation during hypoxia estimated by BOLD MRI in a fetal sheep model. Ultrasound Obstet Gynecol 38(6):665–672
Cannie M, Jani J, De Keyzer F, Roebben I, Dymarkowski S, Deprest J (2009) Diffusion-weighted MRI in lungs of normal fetuses and those with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 34(6):678–686
Avni R, Golani O, Akselrod-Ballin A et al (2016) MR imaging-derived oxygen-hemoglobin dissociation curves and fetal-placental oxygen-hemoglobin affinities. Radiology. 280(1):68–77
Sørensen A, Sinding M, Peters DA et al (2015) Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol Rep 3(10):e12582
Nagao M, Yamasaki Y, Kawanami S et al (2017) Quantification of myocardial oxygenation in heart failure using blood-oxygen-level-dependent T2* magnetic resonance imaging: comparison with cardiopulmonary exercise test. Magn Reson Imaging 39:138–143
Acknowledgments
Analysis of MRI BOLD data was performed at the Life Imaging Facility of Paris Descartes University (Plateforme Imageries du Vivant), supported by France Life Imaging (grant ANR-11-INBS-0006) and Infrastructures Biologie-Santé.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bertand Tavitian, head of the department.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Dr. Afef Bouchouicha).
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective case- observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khen-Dunlop, N., Chalouhi, G., Lecler, A. et al. Assessment of BOLD response in the fetal lung. Eur Radiol 31, 3090–3097 (2021). https://doi.org/10.1007/s00330-020-07272-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07272-z