Abstract
Objectives
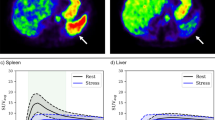
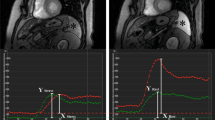
The hepatic arterial buffer response is a mechanism mediated by adenosine whereby hepatic arterial perfusion (HAP) increases when portal flow decreases, and is implicated in liver disease. The first study aim was to measure HAP in patients undergoing myocardial perfusion imaging (MPI), thus developing hepatic arterial rest/stress perfusion imaging (HAPI). The second aim was to compare adenosine-induced changes in splenic perfusion (SP) and HAP with corresponding changes in myocardial blood flow (MBF).
Methods
Patients had MPI with 82Rb PET/CT using adenosine (n = 45) or regadenoson (n = 33) for stressing. SP and HAP were measured using a first-pass technique that gives HAP rather than total hepatic perfusion. Renal perfusion (RP) was also measured.
Results
Mean MBF and HAP increased after both adenosine ([stress-rest]/rest 1.1 and 0.8) and regadenoson (1.4 and 0.6), but the respective changes did not correlate. After adenosine, SP (− 0.48) and RP (− 0.26) both decreased. The change in SP correlated positively with the change in MBF (r = 0.36; p = 0.015) but did not correlate with change in HAP. After regadenoson, SP (0.2) and RP (0.2) both increased. The changes in SP correlated with the changes in MBF (r = 0.39; p = 0.025) and HAP (r = 0.39; p = 0.02). Changes in RP correlated with changes in HAP (r = 0.51; p = 0.0008) but not MBF. Resting SP (r = 0.32; p = 0.004), but not resting HAP, correlated with hepatic fat burden. Adenosine-induced change in HAP also correlated with hepatic fat (r = 0.29; p = 0.05).
Conclusion
HAPI could be a useful new hepatic function test. Neither splenic ‘switch-off’ nor hepatic arterial ‘switch-on’ identifies adequacy of stress in MPI.
Key Points
• This article describes a new method for assessing arterial perfusion of the liver and its capacity to respond to an infusion of adenosine, a substance that normally ‘drives’ hepatic arterial flow.
• Hepatic arterial flow increased in response to adenosine, sometimes dramatically. Adenosine is already used clinically to stimulate myocardial blood flow in patients with suspected coronary disease, but the increase in flow did not correlate with the corresponding increase in hepatic arterial flow.
• Analogous to the use of adenosine in the myocardium, the increase in hepatic arterial flow in response to adenosine has the potential to be a new clinically useful method for the evaluation of hepatic arterial haemodynamics in liver disease.






Similar content being viewed by others
Abbreviations
- CT:
-
Computed tomography
- FDG:
-
Fluorodeoxyglucose
- HABR:
-
Hepatic arterial buffer response
- HAP:
-
Hepatic arterial perfusion
- MBF:
-
Myocardial blood flow
- MPI:
-
Myocardial perfusion imaging
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- RP:
-
Renal perfusion
- SP:
-
Splenic perfusion
References
Lautt WW (2007) Regulatory processes interacting to maintain hepatic blood flow constancy: vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res 37:891–903
Eipel C, Abshagen K, Vollmar B (2010) Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 16:6046–6057
Zhu X, Shiba H, Zhu Y et al (2016) Adenosine increases hepatic artery flow in liver transplant recipients: a pilot study. Transplant Proc 48:116–119
Browse DJ, Mathie RT, Benjamin IS, Alexander B (2003) The role of ATP and adenosine in the control of hepatic blood flow in the rabbit liver in vivo. Comp Hepatol 2(1):9
Manisty C, Ripley DP, Herrey AS et al (2015) Splenic switch-off: a tool to assess stress adequacy in adenosine perfusion cardiac MR imaging. Radiology 276:732–740
Hosking A, Koulouroudias M, Zemrak F et al (2017) Evaluation of splenic switch off in a tertiary imaging centre: validation and assessment of utility. Eur Heart J Cardiovasc Imaging 18:1216–1221
Tarantino G, Scalera A, Finelli C (2013) Liver-spleen axis: intersection between immunity, infections and metabolism. World J Gastroenterol 19:3534–3542
Peters AM, Brown J, Crossman D et al (1990) Non-invasive measurement of renal blood flow with Tc-99m DTPA in the evaluation of patients with suspected renovascular hypertension. J Nucl Med 31:1980–1985
Mathie RT (1986) Hepatic blood flow measurement with inert gas clearance. J Surg Res 41:92–110
Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G (2011) Radiation dosimetry of Rb-82 in humans under pharmacological stress. J Nucl Med 52:485–491
deKemp RA, Declerck J, Klein R et al (2013) Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med 54:571–577
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Da Silva JN, deKemp RA (2007) Quantification of myocardial blood flow with 82Rb. dynamic PET imaging. Eur J Nucl Med Mol Imaging 34:1765–1774
Boyce CJ, Pickhardt PJ, Kim DH et al (2010) Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose AJR Am J Roentgenol 94:623–628
Miles KA, Hayball M, Dixon AK (1991) Colour perfusion imaging: a new application of computed tomography. Lancet 337(8742):643–645
Bell SD, Peters AM (1991) Measurement of blood flow from first-pass radionuclide angiography: influence of bolus volume. Eur J Nucl Med 18:885–888
Choi Y, Huang S-C, Hawkins RA et al (1993) A simplified method for quantification of myocardial blood flow using nitrogen-13-ammonia and dynamic PET. J NucI Med 34:488–497
Bergmann SR (1997) Clinical applications of myocardial perfusion assessments made with oxygen-15 water and positron emission tomography. Cardiology 88:71–79
Holroyd AM, Peters AM (1980) The measurement of arterial perfusion of the liver in man using the radioisotope xenon-133. Br J Surg 67(3):178–180
Hashimoto K, Murakami T, Dono K et al (2007) Quantitative tissue blood flow measurement of the liver parenchyma: comparison between xenon CT and perfusion CT. Dig Dis Sci 52:943–949
Materne R, Smith AM, Peeters F et al (2002) Assessment of hepatic perfusion parameters with dynamic MRI. Magn Reson Med 47:135–142
Tsushima Y, Endo K (2000) Spleen enlargement in patients with nonalcoholic fatty liver: correlation between degree of fatty infiltration in liver and size of spleen. Dig Dis Sci 45:196–200
Keramida G, Dunford A, Kaya G, Anagnostopoulos CD, Peters AM (2018) Hepato-splenic axis: hepatic and splenic metabolic activities are linked. Am J Nucl Med Mol Imaging 8:228–238
Peters AM, Klonizakis I, Lavender JP, Lewis SM (1980) Use of indium-111-labelled platelets to measure spleen function. Br J Haematol 46:587–593
Agmon Y, Dinour D, Brezis M (1993) Disparate effects of adenosine A1- and A2-receptor agonists on intrarenal blood flow. Am J Physiol 265(6):F802–F806
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor A M Peters.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keramida, G., Gregg, S. & Peters, A.M. Stimulation of the hepatic arterial buffer response using exogenous adenosine: hepatic rest/stress perfusion imaging. Eur Radiol 30, 5852–5861 (2020). https://doi.org/10.1007/s00330-020-06984-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06984-6