Abstract
Objectives
To evaluate the prevalence of acute pulmonary embolism (APE) in non-hospitalized COVID-19 patients referred to CT pulmonary angiography (CTPA) by the emergency department.
Methods
From March 14 to April 6, 2020, 72 non-hospitalized patients referred by the emergency department to CTPA for COVID-19 pneumonia were retrospectively identified. Relevant clinical and laboratory data and CT scan findings were collected for each patient. CTPA scans were reviewed by two radiologists to determinate the presence or absence of APE. Clinical classification, lung involvement of COVID-19 pneumonia, and CT total severity score were compared between APE group and non-APE group.
Results
APE was identified in 13 (18%) CTPA scans. The mean age and D-dimer of patients from the APE group were higher in comparison with those from the non-APE group (74.4 vs. 59.6 years, p = 0.008, and 7.29 vs. 3.29 μg/ml, p = 0.011). There was no significant difference between APE and non-APE groups concerning clinical type, COVID-19 pneumonia lung lesions (ground-glass opacity: 85% vs. 97%; consolidation: 69% vs. 68%; crazy paving: 38% vs. 37%; linear reticulation: 69% vs. 78%), CT severity score (6.3 vs. 7.1, p = 0.365), quality of CTPA (1.8 vs. 2.0, p = 0.518), and pleural effusion (38% vs. 19%, p = 0.146).
Conclusions
Non-hospitalized patients with COVID-19 pneumonia referred to CT scan by the emergency departments are at risk of APE. The presence of APE was not limited to severe or critical clinical type of COVID-19 pneumonia.
Key Points
• Acute pulmonary embolism was found in 18% of non-hospitalized COVID-19 patients referred by the emergency department to CTPA. Two (15%) patients had main, four (30%) lobar, and seven (55%) segmental acute pulmonary embolism.
• Five of 13 (38%) patients with acute pulmonary embolism had a moderate clinical type.
• Severity and radiological features of COVID-19 pneumonia showed no significant difference between patients with or without acute pulmonary embolism.
Similar content being viewed by others
Introduction
Non-contrast chest CT has been shown to play an important role in the evaluation of COVID-19 pneumonia [1, 2]. Because of its excellent sensitivity in comparison with the RT-PCR test [3,4,5] and its high specificity in an epidemic context [6], chest CT can be used as a complementary means of diagnosing COVID-19 alongside RT-PCR [2, 7]. Non-contrast chest CT also allows assessing the severity of the pulmonary involvement [8, 9] and could play a key role in therapeutic efficacy evaluation in combination with clinical information [10].
The place of computed tomography pulmonary angiography (CTPA) in the evaluation of COVID-19 pneumonia remains more limited. It is reserved for patients whose clinical condition has deteriorated in order to search for an acute pulmonary embolism (APE) [2]. Recent immunohistologic and biological studies have reported that COVID-19 can cause microvascular injuries and is associated with a procoagulant state [11, 12]. Several published studies seem to confirm the association between COVID-19 and APE in hospitalized patients with severe to critical form [13,14,15,16,17,18]. In our practice, we have found cases of APE in non-hospitalized COVID-19 patients referred to the CTPA by our emergency department.
The objective of our study was to retrospectively assess the prevalence of APE in non-hospitalized COVID-19 patients referred to the CTPA by the emergency department.
Materials and methods
Case selection
All requests for chest CT scans for suspicion or assessment of COVID-19 pneumonia in non-hospitalized patients presenting in the emergency department of our hospital between March 14, 2020, and April 6, 2020, were retrospectively analyzed. Patients diagnosed with COVID-19 who underwent CTPA were included in our study. To have a final diagnosis of COVID-19, patients had to either have at least a positive RT-PCR for SARS-CoV-2, or a combination of compatible clinical findings (fever, cough, dyspnea, asthenia, myalgia, or diarrhea [7]) and a chest CT with typical COVID-19 pneumonia features (bilateral ground-glass opacities with peripheral distribution and vascular thickening [4]). The indications for CTPA varied among emergency physicians and radiologists. They were mainly based on a worsening of the patient’s clinical condition with new onset of dyspnea, desaturation, or chest pain and also an increase in D-dimer levels. Patients were divided into two groups: an APE group for patients with an acute pulmonary embolism discovered by CTPA and a non-APE group for patients without acute pulmonary embolism.
This study was approved by the ethics committee of our institution and the requirement for informed consent was waived.
Clinical and biological data
The following clinical and laboratory data were extracted from our medical records: patient history, age, sex, body mass index (BMI), symptoms (fever (> 37.5 °C), cough, dyspnea, desaturation, chest pain, asthenia, myalgia, and diarrhea), delay between onset of symptoms and CT scan, D-dimer and CRP (C-reactive protein) when available.
Clinical classification
All cases were divided into four groups: minimal, moderate, severe, and critical according to whether there were clinical symptoms, severity of pneumonia, respiratory failure, shock, other organ failure, based on the Diagnosis and Treatment Plan of COVID-19 issued by National Health Commission (7th ed.) (in Chinese) [7]. (1) Mild type: mild clinical symptoms without pneumonia in imaging; (2) moderate type: fever, respiratory tract and other symptoms with pneumonia in imaging; (3) severe type: respiratory distress, respiratory rate ≥ 30 times/min; in resting state, oxygen saturation ≤ 93%; PaO2/FiO2 ≤ 300 mmHg; (4) critical type: respiratory failure requiring mechanical ventilation, shock, and other organ failure requiring ICU monitoring and treatment.
CT acquisition
CT scans were acquired in the supine position on a 64-MDCT (Revolution EVO, GE Healthcare). The CT scanning range covered the area from the apices to the bases of the lungs. The examination began by performing an unenhanced low-dose cranio-caudal series with deep-inspiration breath-holding. The acquisition parameters were as follows: 120 kVp, tube current modulation with a noise index setting of 50 (80–300 mA); ASIR-V, 40%; pitch, 1.531; rotation time, 0.35 s. The examination was completed with a caudal-cranial breath-hold CTPA without deep inspiration in order to avoid a transient interruption of contrast [19], with intravenous administration of 50 ml of iodinated contrast medium at 4 to 5 ml/s followed by a flush of 20 ml of physiological saline solution using a bolus-tracking technique. The CTPA acquisition parameters were as follows: 100 kVp; tube current modulation with a noise index setting of 30 (140–480 mA); ASIR-V, 50%; pitch, 1.531; rotation time, 0.35 s. The images were reconstructed with slice thickness of 1.25 mm and LUNG filter for the unenhanced low-dose series and 0.625 mm and SOFT filter for the CTPA. All images were archived and sent to our institution’s Picture and Archiving Communication System.
Image analysis
All patient images were anonymized and CT scans were randomized for interpretation on SyngoVia post-processing workstations (VB30, Siemens Healthcare). The images were reviewed by two radiologists with 10 and 12 years of experience in thoracic imaging (C.B. and E.P.). The images were reviewed independently with a final decision made by consensus. During image interpretation, the radiologists were able to change the viewing window, to zoom in or out on the images, use multiplanar reformations in any plane, and use maximal intensity projection or minimal intensity projection reformations. They were not made aware of the patient’s clinical data.
For the CTPA images, the evaluation criteria were as follows: quality of the CTPA based on the degree of opacification of the pulmonary arteries and the presence or absence of significant respiratory movement artifacts [20] (1 = excellent quality; 2 = satisfactory quality allowing a correct interpretation of all the pulmonary arteries; 3 = unsatisfactory quality not allowing a correct interpretation of all the pulmonary arteries), presence or not of acute pulmonary embolism (defined by the presence of a defect filling the lumen of a pulmonary artery on at least two consecutive axial sections [21]), location of pulmonary embolism (main, lobar, segmental, or subsegmental pulmonary arteries), topography of pulmonary emboli in the five pulmonary lobes, presence or absence of CT signs of severity (right ventricle/left ventricle ratio of ≥ 1 [22]).
For the lung images, the evaluation criteria were the following: presence or absence of ground-glass opacity (GGO), consolidation, crazy paving, linear opacities (including subpleural curvilinear opacities), and pleural effusion. According to the proportion of each pattern in comparison with the totality of the lung opacification, cases were classified as GGO dominant or consolidation predominant, if the proportion of each one of the patterns was respectively greater than 50% of the total [23]. A quantitative score was used to estimate the pulmonary involvement of all these abnormalities on the basis of the percentage of the total lung involved per lobe. For each of the five lung lobes, the lobar involvement was classified as none (0%), minimal (1–25%), mild (26–50%), moderate (51–75%), or severe (76–100%), with corresponding scores of 0, 1, 2, 3, or 4. The total severity score (TSS) was reached by summing the five lobe scores (range from 0 to 20) [10].
Statistical analysis
Continuous variables were presented as means with standard deviation and extreme values in parentheses and compared by Mann-Whitney U test between APE and non-APE groups. Categorical variables were presented as numbers and percentages and were compared by Fisher’s exact test between both groups. Two-sided p < 0.05 was considered statistically significant. Statistical analysis was done using R for Windows software (version 3.6.3, R Foundation for Statistical Computing).
Results
Patient selection
The results of the patient selection process in our study are shown in Fig. 1. From March, 14, 2020, to April 6, 2020, 211 patients were referred by the emergency department to undergo a chest CT for suspicion or assessment of COVID-19 pneumonia. A total of 146 (69%) patients had a final diagnosis of COVID-19 and 72 (49%) patients underwent CTPA and were included in our study. Thirteen (18%) patients had APE (APE group) versus 59 (82%) without APE (non-APE group). Of the 72 patients included in the study, 58 (80%) patients had a positive RT-PCR for SARS-CoV-2, 10 (14%) did not have RT-PCR (including three from the APE group), and 4 (6%) had a diagnosis based on the typical clinical and radiological presentation of COVID-19 with RT-PCR results negative for SARS-CoV-2 (two patients with a single sample including one patient from the APE group, and two patients with two negative RT-PCR including one from the APE group).
Demographic, clinical, and biological characteristics
Demographic, clinical, and biological characteristics of included patients are summarized in Table 1. The group consisted of 54 men and 18 women with an average age of 62.3 years and an average BMI of 26.7 kg/m2. Patients in the APE group were significantly older than those in the non-APE group (74.4 vs. 59.6 years, p = 0.008) with a significantly lower BMI (24.0 vs. 27.3 kg/m2, p = 0.021). There was no significant difference between the APE group and the non-APE group in terms of male/female distribution and the time between the onset of symptoms and the time at which CTPA was performed (8.3 vs. 7.5 days, p = 0.617). The most frequently found symptoms were fever (71%), dyspnea (68%), and desaturation (67%), with no significant difference between the two groups. Six patients (three in each group) were followed for active neoplasia. The clinical classification type was identified as moderate for 31 (43%) patients and severe to critical for 41 (57%) patients, with no significant difference between both groups. Biologically, 7 (54%) patients in the APE group and 34 (57%) in the non-APE group had a D-dimer test on the same day as the CT scan. A significantly higher D-dimer level was found in the APE group compared with the non-APE group (7.29 vs. 3.29 μg/ml, p = 0.011). Only one patient in the non-APE group had a normal D-dimer level (0.33 μg/ml), and none of those in the APE group. All the patients in the APE group had had a CRP test on the same day as the CT scan as well as 56 (95%) patients in the non-APE group, with no significant difference in CRP levels between the two groups (136 mg/l vs. 105 mg/l, p = 0.407). As of April 15, 2020, 38 (53%) patients had returned home, 23 (32%) were hospitalized, and 11 (15%) had died, with no significant difference in the outcome rate among patients in the APE group compared with the non-APE group.
Imaging findings
Imaging findings of included patients are summarized in Table 2.
Of the 72 CTPAs, 13 (18%) patients presented APE. Two (15%) patients had main, four (30%) lobar, and seven (55%) segmental APE. The segments most often affected were the right lower lobe (61%) and the left lower lobe (54%). Five (38%) patients had bilateral thrombi while eight (62%) had unilateral involvement only. Five (38%) CTPA showed the presence of a CT sign of severity with a RV > LV ratio greater than or equal to 1. The quality score for the interpretation of the CTPA was comparable between the APE and non-APE groups (1.8 vs. 2.0, p = 0.518) with a similar proportion of insufficient quality (38% vs. 35%, p = 1).
Chest CT showed anomalies related to COVID-19 pneumonia in 71 (98.6%) of the 72 patients. One (1.4%) patient in the non-APE group had a normal chest CT scan. No significant difference between the two groups was found in the number of patients with more than two lobes involved (92% vs. 90%, p = 1). There was significantly less involvement of the left upper lobe in the APE group compared with the non-APE group (62% vs. 86%, p = 0.05). The frequency of involvement of the other lobes did not show any significant difference between the two groups. Both the APE and non-APE groups showed similar proportions of GGO (85% vs. 97%, p = 0.147), of consolidation (69% vs. 68%, p = 1), of crazy paving (38% vs. 37%, p = 1), of linear reticulation (69% vs. 78%, p = 0.490), and of pleural effusion (38% vs. 19%, p = 0.146). The type of pulmonary involvement with a predominance of GGO or consolidation was equivalent between the APE and non-APE groups (54% vs. 56%, p = 1; and 46% vs. 42%, p = 1). The TSS did not show a significant difference between the APE and non-APE groups (6.3 vs. 7.1, p = 0.365). Fifty-eight (81%) patients had a TSS lower than 10 with no significant difference between the two groups (85% vs. 80%, p = 1).
Discussion
COVID-19 presents many clinical forms, from cases of pauci- or asymptomatic patients to cases of severe forms of COVID-19 pneumonia which may lead to the patient’s death [24]. The association between COVID-19 pneumonia and APE has already been described in patients hospitalized with severe to critical clinical type [13,14,15,16,17,18]. To the best of our knowledge, no study has yet evaluated the prevalence of APE in outpatients consulting to the emergency department for clinical suspicion or degradation of COVID-19 pneumonia. One case describes the discovery of APE in a COVID-19 patient with a mild clinical form presenting to the emergency department for hemoptysis [25]. In our study, we focused on patients presenting to the emergency department for suspected or worsening COVID-19 pneumonia. Among these patients, many presented not only a deterioration of their clinical state with dyspnea (68%), desaturation (67%), or chest pain (14%) but also an increase in D-dimer levels. These anomalies, although not specific, led us to complement our non-contrast chest CT scans with CTPA to eliminate APE. Of our 146 CT scans performed on COVID-19 patients, we complemented the examinations with 72 (49%) CTPA. Thirteen APE were discovered, representing a prevalence of 18% of the patients included in our study. Eight patients with APE had a severe to critical COVID-19 pneumonia but 5 of the 13 (38%) patients with APE had a moderate clinical form (Fig. 2). These results seem to confirm the association between COVID-19 and APE, even in non-severe and non-hospitalized COVID-19 patients.
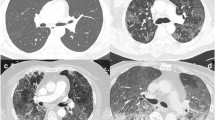
Segmental acute pulmonary embolism in COVID-19 patients: one was a 61-year-old man presenting to the emergency department for fever and myalgia during 9 days with new onset of dyspnea without desaturation. The RT-PCR for SARS-CoV-2 was positive. Unenhanced chest CT scan (a) revealed typical COVID-19 pneumonia with mild lung involvement (TSS of 7). CTPA in axial (b) and coronal reformation (c) showed a segmental acute pulmonary embolism of the right lower lobe (arrows). After 2 days of hospitalization, the patient was discharged at home with a good outcome
Several factors could explain this association. Radiologically, vascular thickening in ground-glass areas has been described in chest CT, which could correspond to a serious inflammatory response with vascular involvement leading to thrombosis [26]. Biologically, several studies have shown that COVID-19 patients tend to have higher D-dimer, fibrinogen, and fibrin degradation product levels [27, 28]. Zhou et al also found in their study that a D-dimer level greater than 1 μg/ml was associated with fatal outcome of COVID-19 [29]. Other factors such as bed rest or confinement could also explain the onset of thromboembolic complications. In our study, three patients with APE had active cancer. Wider use of CTPA for COVID-19 patients seems to be advisable, with particular attention on COVID-19 patients with co-morbidities causing a higher thromboembolic risk.
Given the risks associated with the injection of iodinated contrast medium (renal failure and allergy) and the additional radiation dose due to CTPA, it does not seem reasonable to perform CTPA systematically with COVID-19 patients. In our study, we investigated whether the radiological manifestation of COVID-19 pneumonia in non-contrast chest CT scans could be associated with a higher rate of APE. The TSS of our patients’ CT scans was on average 7/20, with no significant difference between the two groups. Only one (8%) patient in the APE group had a TSS greater than 10 and five (38%) had minimal lung damage with a score lower than 5. As for the TSS, our study did not show any significant difference concerning the type of lung lesions detected in COVID-19 patients with or without APE. Non-contrast chest CT therefore does not make it possible to differentiate the patients requiring complementary CTPA to search for APE. It is worth noting that in our protocol, CTPA was performed using breath-holding apnea without deep inspiration in order to obtain an optimal opacification of the pulmonary arteries by avoiding transient interruption of contrast [19]. But this acquisition without deep inspiration leads to ventilatory disturbances impeding detailed analysis of the pulmonary parenchyma, in particular the GGO. The unenhanced series with deep inspiration therefore remains necessary in order to have an optimal analysis of the pulmonary parenchyma for COVID-19 pneumonia (Fig. 3).
Comparison of lung findings in a COVID-19 patient between unenhanced low-dose chest CT with deep inspiration (a) and CTPA acquisition with breath-hold without deep inspiration (b). The unenhanced series with deep inspiration allows correct analysis of the parenchyma in COVID-19 patients in comparison with CTPA series showing ventilation disturbances which may resemble false GGO images
Although the radiological manifestation of COVID-19 pneumonia does not allow for the selection of patients at risk of APE, we found a significantly higher D-dimer level in the APE group compared with the non-APE group. The D-dimer level seems to be an important parameter in the management of COVID-19 patients, making it possible both to assess the severity of the disease [29] and to suspect APE. Given that the increase in the D-dimer level can be linked to COVID-19, it would be interesting to evaluate on the basis of large-scale studies if there is a cut-off D-dimer level at which CTPA could be recommended to search for APE in COVID-19 patients [16]. Pending further data on D-dimer levels, we believe that all patients with COVID-19 pneumonia and an increased level of D-dimer should benefit from CTPA to eliminate APE, whenever possible.
Our study had several limitations. First, the number of patients included was small. Other large-scale studies are needed to confirm our results and analyze whether other factors could help optimize the indications for CTPA in COVID-19 patients. Second, certain medical or biological data were not available due to the retrospective nature of our study. Third, our study did not include a control group to compare the prevalence of APE in a group of patients with COVID-19 pneumonia versus other types of pneumonia. Fourth, no venous doppler ultrasound was performed to assess deep vein thrombosis that could explain the etiopathogenesis of APE in some cases. Finally, the CTPA quality scores showed that 36% of CT scans had an interpretation score of 3, i.e., not optimal for the analysis of all the pulmonary arteries. This was mainly due to respiratory artifacts in patients with dyspnea. It is therefore difficult to totally exclude the possibility that some patients may have had distal APE not seen in the CTPA.
In conclusion, our study showed an 18% prevalence of APE in non-hospitalized COVID-19 patients referred to CTPA by the emergency department. COVID-19 patients with APE were older and had higher D-dimer levels. No significant difference was found in the severity of pulmonary involvement in CT. More studies are needed to determine which COVID-19 patients require CTPA as a complement to the non-contrast chest CT scan.
Abbreviations
- APE:
-
Acute pulmonary embolism
- COVID-19:
-
Coronavirus disease 19
- CTPA:
-
Computed tomography pulmonary angiography
- GGO:
-
Ground-glass opacity
- RT-PCR:
-
Reverse transcription polymerase chain reaction
References
Yang W, Sirajuddin A, Zhang X et al (2020) The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol. https://doi.org/10.1007/s00330-020-06827-4
Revel M-P, Parkar AP, Prosch H et al (2020) COVID-19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. https://doi.org/10.1007/s00330-020-06865-y
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. https://doi.org/10.1148/radiol.2020200642
Wen Z, Chi Y, Zhang L et al (2020) Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects. Radiology: Cardiothorac Imaging. https://doi.org/10.1148/ryct.2020200092
Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. https://doi.org/10.1148/radiol.20200200432
Bai HX, Hsieh B, Xiong Z et al (2020) Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. https://doi.org/10.1148/radiol.20200200823
General Office of National Health Committee. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial revised fifth edition). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed 15 Apr 2020
Yang R, Lo X, Liu H et al (2020) Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiology: Cardiothorac Imaging. https://doi.org/10.1148/ryct.2020200047
Zhou Z, Guo D, Li C et al (2020) Coronavirus disease 2019: initial chest CT findings. Eur Radiol. https://doi.org/10.1007/s00330-020-06816-7
Li K, Fang Y, Li W et al (2020) CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. https://doi.org/10.1007/s00330-020-06817-6
Magro C, Mulvey JJ, Berlin D et al (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. https://doi.org/10.1016/j.trsl.2020.04.007
Ranucci M, Ballotta A, Di Dedda U et al (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. https://doi.org/10.1111/jth.14854
Danzi GB, Loffi M, Galeazzi G, Gherbesi E (2020) Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa254
Xie Y, Wang X, Yang P, Zhang S (2020) COVID-19 complicated by acute pulmonary embolism. Radiology: Cardiothorac Imaging. https://doi.org/10.1148/ryct.2020200067
Klok FA, Kruip MJHA, van der Meer NJM et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. https://doi.org/10.1016/j.thromres.2020.04.013
Leonard-Lorant I, Delabranche X, Severac F et al (2020) Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. https://doi.org/10.1148/radiol.2020201561
Grillet F, Behr J, Calame P, Aubry S, Delabrousse E (2020) Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. https://doi.org/10.1148/radiol.2020201544
Chen J, Wang X, Zhang S et al (2020) Findings of acute pulmonary embolism in COVID-19 patients. Available at SSRN: https://ssrn.com/abstract=3548771 or 102139/ssrn3548771Google Scholar
Sudarski S, Haubenreisser H, Henzler T et al (2019) Incidence of transient interruption of contrast (TIC) - a retrospective single-centre analysis in CT pulmonary angiography exams acquired during inspiratory breath-hold with the breathing command: “please inspire gently!”. PLoS One 14:e0210473
Browne AM, Cronin CG, English C, NiMhuircheartaigh J, Murphy JM, Bruzzi JF (2010) Unsuspected pulmonary emboli in oncology patients undergoing routine computed tomography imaging. J Thorac Oncol 5:798–803
Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC (2004) CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics 24:1219–1238
Meinel FG, Nance JW Jr, Schoepf UJ et al (2015) Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med 128:747–59.e2
Ng M-Y, Lee EYP, Yang J et al (2020) Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothorac Imaging. https://doi.org/10.1148/ryct.2020200034
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Casey K, Iteen A, Nicolini R, Auten J (2020) COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2020.04.011
Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.20.22976
Lippi G, Favaloro EJ (2020) D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. https://doi.org/10.1055/s-0040-1709650
Han H, Yang L, Liu R et al (2020) Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2020-0188
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. GERVAISE Alban.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (GERVAISE Alban) has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of the retrospective design of the study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gervaise, A., Bouzad, C., Peroux, E. et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol 30, 6170–6177 (2020). https://doi.org/10.1007/s00330-020-06977-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06977-5