Abstract
Objective
Little is known about the prevalence and degree of deformation of surgically implanted aortic biological valve prostheses (bio-sAVRs). We assessed bio-sAVR deformation using multidetector-row computed tomography (MDCT).
Methods
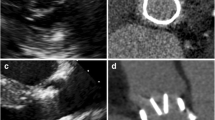
Three imaging databases were searched for patients with MDCT performed after bio-sAVR implantation. Minimal and maximal valve ring diameters were obtained in systole and/or diastole, depending on the acquired cardiac phase(s). The eccentricity index (EI) was calculated as a measure of deformation as (1 − (minimal diameter/maximal diameter)) × 100%. EI of < 5% was considered none or trivial deformation, 5–10% mild deformation, and > 10% non-circular. Indications for MDCT and implanted valve type were retrieved.
Results
One hundred fifty-two scans of bio-sAVRs were included. One hundred seventeen measurements were performed in systole and 35 in diastole. None or trivial deformation (EI < 5%) was seen in 67/152 (44%) of patients. Mild deformation (EI 5–10%) was seen in 59/152 (39%) and non-circularity was found in 26/152 (17%) of cases. Overall, median EI was 5.5% (IQR 3.4–7.8). In 77 patients, both systolic and diastolic measurements were performed from the same scan. For these scans, the median EI was 6.5% (IQR 3.4–10.2) in systole and 5.1% (IQR3.1–7.6) in diastole, with a significant difference between both groups (p = 0.006).
Conclusions
Surgically implanted aortic biological valve prostheses show mild deformation in 39% of cases and were considered non-circular in 17% of studied valves.
Key Points
• Deformation of surgically implanted aortic valve bioprostheses (bio-sAVRs) can be adequately assessed using MDCT.
• Bio-sAVRs show at least mild deformation (eccentricity index > 5%) in 56% of studied cases and were considered non-circular (eccentricity index > 10%) in 17% of studied valves.
• The higher deformity rate found in bio-sAVRs with (suspected) valve pathology could suggest that geometric deformity may play a role in leaflet malformation and thrombus formation similar to that of transcatheter heart valves.


Similar content being viewed by others
Abbreviations
- AVR:
-
Aortic valve replacement
- Bio-sAVR:
-
Biological surgical aortic valve replacement
- EI:
-
Eccentricity index
- IQR:
-
Interquartile range
- MDCT:
-
Multidetector-row computed tomography
- sAVR:
-
Surgical aortic valve replacement
- SVD:
-
Structural valve deterioration
- TAVI:
-
Transcatheter aortic valve implantation
- THV:
-
Transcatheter heart valve
References
Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS (2009) Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 137:82–90
Samad Z, Vora AN, Dunning A et al (2016) Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J 37:2276–2286
Côté N, Pibarot P, Clavel MA (2017) Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr Opin Cardiol 32:123–129
Hoffmann G, Lutter G, Cremer J (2008) Durability of bioprosthetic cardiac valves. Dtsch Arztebl Int 105:143–148
Binder RK, Webb JG, Toggweiler S et al (2013) Impact of post-implant Sapien XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv 6:462–468
Delgado V, Ng AC, van de Veire NR et al (2010) Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 31:1114–1123
Willson AB, Webb JG, Gurvitch R et al (2012) Structural integrity of balloon-expandable stents after transcatheter aortic valve replacement: assessment by multidetector computed tomography. JACC Cardiovasc Interv 5:525–532
Fuchs A, De Backer O, Brooks M et al (2017) Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 13:1067–1075
Caudron J, Fares J, Hauville C et al (2011) Evaluation of multislice computed tomography early after transcatheter aortic valve implantation with the Edwards Sapien bioprosthesis. Am J Cardiol 108:873–881
Schultz CJ, Weustink A, Piazza N et al (2009) Geometry and degree of apposition of the corevalve revalving system with multislice computed tomography after implantation in patients with aortic stenosis. J Am Coll Cardiol 54:911–918
Wood DA, Tops LF, Mayo JR et al (2009) Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 103:1295–1301
Abbasi M, Azadani AN (2015) Leaflet stress and strain distributions following incomplete transcatheter aortic valve expansion. J Biomech 48:3663–3671
Rodríguez-Olivares R, Rahhab Z, El Faquir N et al (2016) Differences in frame geometry between balloon-expandable and self-expanding transcatheter heart valves and association with aortic regurgitation. Rev Esp Cardiol (Engl Ed) 69:392–400
Schoen FJ, Levy RJ (2005) Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 79:1072–1080
Thubrikar MJ, Deck JD, Aouad J, Nolan SP (1983) Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg 86:115–125
Sritharan D, Fathi P, Weaver JD, Retta SM, Wu C, Duraiswamy N (2018) Impact of clinically relevant elliptical deformations on the damage patterns of sagging and stretched leaflets in a bioprosthetic heart valve. Cardiovasc Eng Technol 9:351–364
Bernacca GM, Fisher AC, Wilkinson R, Mackay TG, Wheatley DJ (1992) Calcification and stress distribution in bovine pericardial heart valves. J Biomed Mater Res 26:959–966
Smith DB, Sacks MS, Pattany PM, Schroeder R (1999) Fatigue-induced changes in bioprosthetic heart valve three-dimensional geometry and the relation to tissue damage. J Heart Valve Dis 8:25–33
van Nooten G, Ozaki S, Herijgers P, Segers P, Verdonck P, Flameng W (1999) Distortion of the stentless porcine valve induces accelerated leaflet fibrosis and calcification in juvenile sheep. J Heart Valve Dis 8:34–41
Binder RK, Rodes-Cabau J, Wood DA et al (2013) Transcatheter aortic valve replacement with the Sapien 3: a new balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv 6:293–300
Blanke P, Reinohl J, Schlensak C et al (2012) Prosthesis oversizing in balloon-expandable transcatheter aortic valve implantation is associated with contained rupture of the aortic root. Circ Cardiovasc Interv 5:540–548
Willson AB, Rodes-Cabau J, Wood DA et al (2012) Transcatheter aortic valve replacement with the St. Jude medical portico valve: first-in-human experience. J Am Coll Cardiol 60:581–586
Haziza F, Papouin G, Barratt-Boyes B, Christie G, Whitlock R (1996) Tears in bioprosthetic heart valve leaflets without calcific degeneration. J Heart Valve Dis 5:35–39
Vesely I, Barber JE, Ratliff NB (2001) Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J Heart Valve Dis 10:471–477
Schoen FJ, Levy RJ (1999) Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res 47:439–465
de Heer LM, Budde RP, van Prehn J et al (2012) Pulsatile distention of the nondiseased and stenotic aortic valve annulus: analysis with electrocardiogram-gated computed tomography. Ann Thorac Surg 93:516–522
Sucha D, Tuncay V, Prakken NH et al (2015) Does the aortic annulus undergo conformational change throughout the cardiac cycle? A systematic review. Eur Heart J Cardiovasc Imaging 16:1307–1317
Suchá D, Daans CG, Symersky P et al (2015) Reliability, agreement, and presentation of a reference standard for assessing implanted heart valve sizes by multidetector-row computed tomography. Am J Cardiol 116:112–120
Ac N, Delgado V, van der Kley F et al (2010) Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 3:94–102
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ricardo Budde, Erasmus MC, Rotterdam.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was not required because the acquisition was a part of the routine clinical workup, and data were gathered retrospectively. No additional acquisitions were made specifically for this study. Patient data were retrieved from the electronic patient file.
Methodology
• retrospective
• observational
• performed at three institutions
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marguerite E. Faure and Dominika Suchá contributed equally to this work.
Electronic supplementary material
Video 1
Manually deforming the stent frame of a bio-sAVR: The video demonstrates how most bio-sAVR stent frames can easily be deformed by external forces. (MP4 1636 kb)
Video 2
In retrospectively scanned patients, a video of the bio-sAVR shows the movement of the valve during all cardiac phases. (MP4 2506 kb)
Rights and permissions
About this article
Cite this article
Faure, M.E., Suchá, D., Schwartz, F.R. et al. Surgically implanted aortic valve bioprostheses deform after implantation: insights from computed tomography. Eur Radiol 30, 2651–2657 (2020). https://doi.org/10.1007/s00330-019-06634-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06634-6