Abstract
Objective
Pancreatitis often represents a continuous inflammatory process, from the first episode of acute pancreatitis (FAP) to recurrent acute pancreatitis (RAP) to chronic pancreatitis (CP). Psoas muscle size is a validated surrogate for global skeletal mass, changes in which are associated with inflammation. The objective was to investigate psoas muscle size in individuals following FAP, RAP, and CP, as well as its associations with pro-inflammatory cytokines.
Methods
Individuals following pancreatitis and healthy individuals were recruited. All participants underwent magnetic resonance imaging, from which psoas muscle volume was derived independently by two raters in a blinded fashion. Circulating levels of four major cytokines (interleukin-6, tumour necrosis factor-α, C-C motif chemokine ligand 2, and leptin) were measured. Five linear regression additive models were built to adjust for possible confounders (age, sex, body composition, physical activity, tobacco smoking, alcohol consumption, comorbidities, and endocrine and exocrine pancreatic functions).
Results
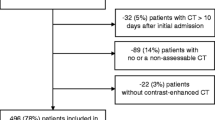
A total of 145 participants were enrolled. A significant downward trend in psoas muscle volume was observed between healthy controls and individuals following FAP, RAP, and CP in all adjusted models (p = 0.047, 0.005, 0.004, and < 0.001). Leptin was significantly associated with psoas muscle volume in all models (β = − 0.16, p = 0.030 in the most adjusted model). The other studied cytokines were not significantly associated with psoas muscle volume.
Conclusions
Psoas muscle size is significantly reduced along the continuum from FAP to RAP to CP. Leptin appears to be one of the factors implicated in this. Further studies are warranted to investigate the relationship between skeletal muscle and inflammation of the pancreas.
Key Points
• First acute pancreatitis, recurrent acute pancreatitis, and chronic pancreatitis were associated with progressively reduced psoas muscle size.
• The findings were independent of age, sex, body fat composition, physical activity, tobacco smoking, alcohol consumption, comorbidities, and exocrine and endocrine functions of the pancreas.
• The mechanism underlying the observed findings may involve hyperleptinaemia.


Similar content being viewed by others
Abbreviations
- ACCI:
-
Age-adjusted Charlson comorbidity index
- BMI:
-
Body mass index
- CCL2:
-
C-C motif chemokine ligand 2
- CI:
-
Confidence interval
- CP:
-
Chronic pancreatitis
- FAP:
-
First episode of acute pancreatitis
- HbA1c:
-
Glycated haemoglobin
- ICC:
-
Intraclass correlation coefficient
- IL-6:
-
Interleukin 6
- IQR:
-
Interquartile range
- PMI:
-
Psoas muscle index
- PMV:
-
Psoas muscle volume
- RAP:
-
Recurrent acute pancreatitis
- TNFα:
-
Tumour necrosis factor-α
- SFV:
-
Subcutaneous fat volume
- VFV:
-
Visceral fat volume
- V/S:
-
Visceral to subcutaneous (fat volume ratio)
References
Xiao AY, Tan ML, Wu LM et al (2016) Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 1:45–55
Pendharkar SA, Mathew J, Petrov MS (2017) Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: a population-based study. Dig Liver Dis 49:540–544
Guda NM, Muddana V, Whitcomb DC et al (2018) Recurrent acute pancreatitis: international state-of-the-science conference with recommendations. Pancreas 47:653–666
Sankaran S, Xiao A, Wu L, Windsor J, Forsmark C, Petrov MS (2015) Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 149:1490–1500.e1
Cho J, Scragg R, Pandol SJ, Goodarzi MO, Petrov MS (2019) Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: a nationwide cohort study. Diabetes Care 42:1675–1683
DeSouza SV, Priya S, Cho J, Singh RG, Petrov MS (2019) Pancreas shrinkage following recurrent acute pancreatitis: an MRI study. Eur Radiol 29:3746–3756
Petrov MS, Yadav D (2019) Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 16:175–184
Heberton G, Nassif M, Bierhals A et al (2016) Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol 118:1363–1367
Carey E, Lai J, Wang C et al (2017) A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 23:625–633
Faron A, Pieper CC, Schmeel FC et al (2019) Fat-free muscle area measured by magnetic resonance imaging predicts overall survival of patients undergoing radioembolization of colorectal cancer liver metastases. Eur Radiol 29:4709–4717
Moorthi R, Avin K (2017) Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 26:219–228
Carrara G, Pecorelli N, De Cobelli F et al (2017) Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr 36:1649–1653
Stretch C, Aubin J, Mickiewicz B et al (2018) Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One 13:e0196235
Pecorelli N, Capretti G, Sandini et al (2017) Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomy for cancer: results from a multicenter study. Ann Surg Oncol 25:308–317
Sandini M, Bernasconi D, Fior D et al (2016) A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition 32:1231–1237
Joglekar S, Asghar A, Mott SL et al (2015) Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 111:771–775
Onesti J, Wright G, Kenning S et al (2016) Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology 16:284–289
Jang M, Park HW, Huh J et al (2019) Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol 29:2417–2425
Noguchi H, Miyasaka Y, Kaku K et al (2018) Preoperative muscle volume predicts graft survival after pancreas transplantation: a retrospective observational cohort study. Transplant Proc 50:1482–1488
Yoon S, Choi M, Lee I et al (2017) Impact of body fat and muscle distribution on severity of acute pancreatitis. Pancreatology 17:188–193
Olesen S, Büyükuslu A, Køhler M, Rasmussen H, Drewes A (2019) Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology 19:245–251
Bano G, Trevisan C, Carraro S et al (2017) Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 96:10–15
Petrov MS (2019) Skeletal muscle: a new piece in the pancreatitis puzzle. United European Gastroenterol J 7:1283–1284
Boutin RD, Yao L, Canter RJ, Lenchik L (2015) Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol 205:W255–W266
Takayama K, Kita T, Nakamura H et al (2016) New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine (Phila Pa 1976) 41:E84–E90
Tosato M, Marzetti E, Cesari M et al (2017) Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res 29:19–27
Sergi G, Trevisan C, Veronese N, Lucato P, Manzato E (2016) Imaging of sarcopenia. Eur J Radiol 85:1519–1520
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205
Amini N, Spolverato G, Gupta R et al (2015) Impact Total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 19:1593–1602
Cervantes A, Singh RG, Kim J, DeSouza SV, Petrov MS (2019) Relationship of anthropometric indices to abdominal body composition: a multi-ethnic New Zealand magnetic resonance imaging study. J Clin Med Res 11:435–446
Singh RG, Cervantes A, Kim J et al (2019) Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 316:G806–G815
Singh RG, Nguyen N, Cervantes A, Alarcon Ramos G, Cho J, Petrov MS (2019) Associations between intra-pancreatic fat deposition and circulating levels of cytokines. Cytokine 120:107–114
Singh RG, Nguyen N, Cervantes A, Cho J, Petrov MS (2019) Serum lipid profile as a biomarker of intra-pancreatic fat deposition: a nested cross-sectional study. Nutr Metab Cardiovasc Dis 29:956–964
Ebbeling L, Grabo DJ, Shashaty M et al (2013) Psoas:lumbar vertebra index: central sarcopenia independently predicts morbidity in elderly trauma patients. Eur J Trauma Emerg Surg 40:57–65
Singh RG, Nguyen N, Cervantes A, Kim J, Stuart CE, Petrov MS (2019) Circulating levels of lipocalin-2 are associated with fatty pancreas but not fatty liver. Peptides 119:170117
Singh RG, Nguyen NN, DeSouza SV, Pendharkar SA, Petrov MS (2019) Comprehensive analysis of body composition and insulin traits associated with intra-pancreatic fat deposition in healthy individuals and people with new-onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes Metab 21:417–423
Stuart CE, Singh RG, Alarcon Ramos GC et al (2020) Relationship of pancreas volume to tobacco smoking and alcohol consumption following pancreatitis. Pancreatology 20:60–67
Issa Y, Kempeneers M, van Santvoort H, Bollen T, Bipat S, Boermeester M (2017) Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol 27:3820–3844
Guerra B, Santana A, Fuentes T et al (2007) Leptin receptors in human skeletal muscle. J Appl Physiol (1985) 102:1786–1792
Singh RG, Pendharkar SA, Gillies NA, Miranda-Soberanis V, Plank LD, Petrov MS (2017) Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin Exp Med 17:477–487
Kim H, Kim M, Kojima N et al (2016) Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc 17:1011–1019
Santoro A, Guidarelli G, Ostan R et al (2019) Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur Radiol 29:4968–4979
Manoy P, Anomasiri W, Yuktanandana P et al (2017) Elevated serum leptin levels are associated with low vitamin D, sarcopenic obesity, poor muscle strength, and physical performance in knee osteoarthritis. Biomarkers 22:723–730
Sconfienza LM (2019) Sarcopenia: ultrasound today, smartphones tomorrow? Eur Radiol 29:1–2
Wolsk E, Mygind H, Grøndahl T, Pedersen B, van Hall G (2012) Human skeletal muscle releases leptin in vivo. Cytokine 60:667–673
Sáinz N, Rodríguez A, Catalán V et al (2012) Leptin reduces the expression and increases the phosphorylation of the negative regulators of GLUT4 traffic TBC1D1 and TBC1D4 in muscle of ob/ob mice. PLoS One 7:e29389
Maden-Wilkinson T, McPhee J, Rittweger J, Jones D, Degens H (2013) Thigh muscle volume in relation to age, sex and femur volume. Age (Dordr) 36:383–393
Xiao AY, Tan ML, Plana MN, Yadav D, Zamora J, Petrov MS (2018) The use of international classification of diseases codes to identify patients with pancreatitis: a systematic review and meta-analysis of diagnostic accuracy studies. Clin Transl Gastroenterol 9:191
Li C, Yu K, Shyh-Chang N et al (2019) Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 10:586–600
Verlaan S, Aspray T, Bauer J et al (2017) Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr 36:267–274
Hopkinson NS, Tennant RC, Dayer MJ et al (2007) A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res 8:25
Bharmal SH, Pendharkar S, Singh RG, Cho J, Petrov MS (2019) Glucose counter-regulation after acute pancreatitis. Pancreas 48:670–681
Martone A, Marzetti E, Calvani R et al (2017) Exercise and protein intake: a synergistic approach against sarcopenia. Biomed Res Int 2017:1–7
Duggan S, Smyth N, O’Sullivan M, Feehan S, Ridgway P, Conlon K (2014) The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract 29:348–354
Cho J, Scragg R, Petrov MS (2019) Use of insulin and the risk of progression of pancreatitis: a population-based cohort study. Clin Pharmacol Ther. https://doi.org/10.1002/cpt.1644
Petrov MS (2019) Metabolic trifecta after pancreatitis: exocrine pancreatic dysfunction, altered gut microbiota, and new-onset diabetes. Clin Transl Gastroenterol 10:e00086
Acknowledgements
This study was part of the Clinical and epidemiOlogical inveStigations in Metabolism, nutritiOn, and pancreatic diseaseS (COSMOS) program.
Funding
COSMOS is supported, in part, by the Royal Society of New Zealand (Rutherford Discovery Fellowship to Associate Professor Max Petrov).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Associate Professor Max Petrov, MD, MPH, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors have significant statistical expertise.
Informed consent
Written informed consent was obtained from all participants in this study.
Ethical approval
Health and Disability Ethics Committee approval was obtained.
Methodology
• Prospective
• Cross-sectional study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 498 kb)
Rights and permissions
About this article
Cite this article
Modesto, A.E., Stuart, C.E., Cho, J. et al. Psoas muscle size as a magnetic resonance imaging biomarker of progression of pancreatitis. Eur Radiol 30, 2902–2911 (2020). https://doi.org/10.1007/s00330-019-06633-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06633-7