Abstract
Objective
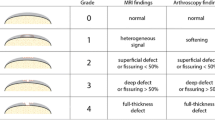
To assess the performance of a morphological evaluation, based on a clinically relevant magnetic resonance imaging (MRI) protocol, in scoring the severity of knee cartilage damage. Specifically, to evaluate the reproducibility, repeatability, and agreement of MRI evaluation with the gross pathology examination (GPE) of the tissue.
Methods
MRI of the knee was performed the day before surgery in 23 patients undergoing total knee arthroplasty. Osteochondral tissue resections were collected and chondral defects were scored by GPE according to a semi-quantitative scale. MR images were independently scored by four radiologists, who assessed the severity of chondral damage according to equivalent criteria. Inter- and intra-rater agreements of MRI evaluations were assessed. Correlation, precision, and accuracy metrics between MRI and GPE scores were calculated.
Results
Moderate to substantial inter-rater agreement in scoring cartilage damage by MRI was found among radiologists. Intra-rater agreement was higher than 96%. A significant positive monotonic correlation between GPE and MRI scores was observed for all radiologists, although higher correlation values were obtained by radiologists with expertise in musculoskeletal radiology and/or longer experience. The accuracy of MRI scores displayed a spatial pattern, characterized by lesion overestimation in the lateral condyle and underestimation in the medial condyle with respect to GPE.
Conclusions
Evaluation of knee cartilage morphology by MRI is a reproducible and repeatable technique, which positively correlates with GPE. Clinical expertise in musculoskeletal radiology positively impacts the evaluation reliability. These findings may help to address limitations in MRI evaluation of knee chondral lesions, thus improving MRI assessment of knee cartilage.
Key Points
• MRI evaluation of knee cartilage shows moderate to strong correlation with gross pathology examination.
• MRI evaluation overestimates cartilage damage in the lateral condyle and underestimates it in the medial condyle.
• Education and experience of the radiologist play a role in MRI evaluation of knee chondral lesions.





Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- AD:
-
Absolute difference
- FSE:
-
Fast spin echo
- GPE:
-
Gross pathology examination
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- RD:
-
Relative difference
- TKA:
-
Total knee arthroplasty
- TRUFI 3D:
-
Three-dimensional true fast imaging with steady-state free precession
References
Recht M, Bobic V, Burstein D et al (2001) Magnetic resonance imaging of articular cartilage. Clin Orthop 391:S379–S396. https://doi.org/10.1097/00003086-200110001-00035
Recht MP, Goodwin DW, Winalski CS, White LM (2005) MRI of articular cartilage: revisiting current status and future directions. AJR Am J Roentgenol 185:899–914. https://doi.org/10.2214/AJR.05.0099
Link TM, Stahl R, Woertler K (2007) Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol 17:1135–1146. https://doi.org/10.1007/s00330-006-0453-5
Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK (2009) Recent advances in MRI of articular cartilage. AJR Am J Roentgenol 193:628–638. https://doi.org/10.2214/AJR.09.3042
Burstein D, Gray M, Mosher T, Dardzinski B (2009) Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am 47:675–686. https://doi.org/10.1016/j.rcl.2009.04.003
Wei H, Lin H, Qin L et al (2019) Quantitative susceptibility mapping of articular cartilage in patients with osteoarthritis at 3T. J Magn Reson Imaging 49:1665–1675. https://doi.org/10.1002/jmri.26535
Stahl R, Luke A, Li X et al (2009) T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients–a 3.0-tesla MRI study. Eur Radiol 19:132–143. https://doi.org/10.1007/s00330-008-1107-6
Hangaard S, Gudbergsen H, Daugaard CL et al (2018) Delayed gadolinium-enhanced MRI of menisci and cartilage (dGEMRIM/dGEMRIC) in obese patients with knee osteoarthritis: crosssectional study of 85 obese patients with intra-articular administered gadolinium contrast. J Magn Reson Imaging 48:1700–1706. https://doi.org/10.1002/jmri.26190
Li X, Pedoia V, Kumar D et al (2015) Cartilage T1ρ and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage 23:2214–2223. https://doi.org/10.1016/j.joca.2015.07.006
Brinkhof S, Nizak R, Khlebnikov V, Prompers JJ, Klomp DWJ, Saris DBF (2018) Detection of early cartilage damage: feasibility and potential of gagCEST imaging at 7T. Eur Radiol 28:2874–2881. https://doi.org/10.1007/s00330-017-5277-y
Duarte A, Ruiz A, Ferizi U et al (2019) Diffusion tensor imaging of articular cartilage using a navigated radial imaging spin-echo diffusion (RAISED) sequence. Eur Radiol 29:2598–2607. https://doi.org/10.1007/s00330-018-5780-9
Tsai P-H, Wong C-C, Chan WP, Lu T-W (2019) The value of MR T2* measurements in normal and osteoarthritic knee cartilage: effects of age, sex, and location. Eur Radiol 29:4514–4522. https://doi.org/10.1007/s00330-018-5826-z
Link TM (2018) Editorial comment: the future of compositional MRI for cartilage. Eur Radiol 28:2872–2873. https://doi.org/10.1007/s00330-018-5457-4
Truhn D, Sondern B, Oehrl S et al (2019) Differentiation of human cartilage degeneration by functional MRI mapping-an ex vivo study. Eur Radiol. https://doi.org/10.1007/s00330-019-06283-9
Roemer FW, Kijowski R, Guermazi A (2017) Editorial: from theory to practice - the challenges of compositional MRI in osteoarthritis research. Osteoarthritis Cartilage 25:1923–1925. https://doi.org/10.1016/j.joca.2017.08.007
Hayashi D, Li X, Murakami AM et al (2018) Understanding magnetic resonance imaging of knee cartilage repair: a focus on clinical relevance. Cartilage 9:223–236. https://doi.org/10.1177/1947603517710309
Haj-Mirzaian A, Guermazi A, Pishgar F et al (2019) Patellofemoral morphology measurements and their associations with tibiofemoral osteoarthritis-related structural damage: exploratory analysis on the osteoarthritis initiative. Eur Radiol. https://doi.org/10.1007/s00330-019-06324-3
Sun D, Neumann J, Joseph GB et al (2019) Introduction of an MR based semi-quantitative score for assessing partial meniscectomy and relation to knee joint degenerative disease: data from the osteoarthritis initiative. Eur Radiol 29:3262–3272. https://doi.org/10.1007/s00330-018-5924-y
Kornaat PR, Ceulemans RYT, Kroon HM et al (2005) MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)–inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol 34:95–102. https://doi.org/10.1007/s00256-004-0828-0
Peterfy CG, Guermazi A, Zaim S et al (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190. https://doi.org/10.1016/j.joca.2003.11.003
Hunter DJ, Lo GH, Gale D et al (2008) The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds osteoarthritis knee score). Ann Rheum Dis 67:206–211. https://doi.org/10.1136/ard.2006.066183
Hunter DJ, Guermazi A, Lo GH et al (2011) Evolution of semi quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage 19:990–1002. https://doi.org/10.1016/j.joca.2011.05.004
Marlovits S, Striessnig G, Resinger CT et al (2004) Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol 52:310–319. https://doi.org/10.1016/j.ejrad.2004.03.014
Marlovits S, Singer P, Zeller P et al (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23. https://doi.org/10.1016/j.ejrad.2005.08.007
Roemer FW, Guermazi A, Trattnig S et al (2014) Whole joint MRI assessment of surgical cartilage repair of the knee: cartilage repair osteoarthritis knee score (CROAKS). Osteoarthritis Cartilage 22:779–799. https://doi.org/10.1016/j.joca.2014.03.014
Link TM, Steinbach LS, Ghosh S et al (2003) Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 226:373–381. https://doi.org/10.1148/radiol.2262012190
Yoshioka H, Stevens K, Hargreaves BA et al (2004) Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging 20:857–864. https://doi.org/10.1002/jmri.20193
Bredella MA, Tirman PF, Peterfy CG et al (1999) Accuracy of T2-weighted fast spin-echoMR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol 172:1073–1080. https://doi.org/10.2214/ajr.172.4.10587150
Bittersohl B, Mamisch TC, Welsch GH et al (2009) Experimental model to evaluate in vivo and in vitro cartilage MR imaging by means of histological analyses. Eur J Radiol 70:561–569. https://doi.org/10.1016/j.ejrad.2008.02.031
Calvo E, Palacios I, Delgado E et al (2004) Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthritis Cartilage 12:878–886. https://doi.org/10.1016/j.joca.2004.07.007
Trattnig S, Huber M, Breitenseher MJ et al (1998) Imaging articular cartilage defects with 3D fat-suppressed echo planar imaging: comparison with conventional 3D fat-suppressed gradient echo sequence and correlation with histology. J Comput Assist Tomogr 22:8–14
McGibbon CA, Trahan CA (2003) Measurement accuracy of focal cartilage defects from MRI and correlation of MRI graded lesions with histology: a preliminary study. Osteoarthritis Cartilage 11:483–493
Li X, Cheng J, Lin K et al (2011) Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 29:324–334. https://doi.org/10.1016/j.mri.2010.09.004
Viera AJ, Garrett JM (2005) Understanding interobserver agreement:the kappa statistic. Fam Med 37:360–363
Rubenstein JD, Li JG, Majumdar S, Henkelman RM (1997) Image resolution and signal-to-noise ratio requirements forMR imaging of degenerative cartilage. AJR Am J Roentgenol 169:1089–1096. https://doi.org/10.2214/ajr.169.4.9308470
Kornaat PR, Doornbos J, van der Molen AJ et al (2004) Magnetic resonance imaging of knee cartilage using a water selective balanced steady-state free precession sequence. J Magn Reson Imaging 20:850–856. https://doi.org/10.1002/jmri.20194
Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47:487–504
Duc SR, Pfirrmann CWA, Schmid MR et al (2007) Articular cartilage defects detected with 3D water-excitation true FISP:prospective comparison with sequences commonly used for knee imaging. Radiology 245:216–223. https://doi.org/10.1148/radiol.2451060990
Chen CA, Kijowski R, Shapiro LM et al (2010) Cartilage morphology at 3.0T: assessment of three-dimensional magnetic resonance imaging techniques. J Magn Reson Imaging 32:173–183. https://doi.org/10.1002/jmri.22213
Acknowledgments
The authors are grateful to the technical staff of the Division of Diagnostic Radiology at Rovereto Hospital for assistance in collecting MR data.
Funding
The study has been supported in part by the Healthcare Research and Innovation Program (IRCS) of the Autonomous Province of Trento (Healthcare Research and Innovation Program IRCS-HTA 2016) and by the Fondazione Cassa di Risparmio di Trento e Rovereto (EviVa project, 2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Sabino Walter Della Sala.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (MR) has significant statistical expertise (got a PhD in biomedical statistics).
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marinetti, A., Tessarolo, F., Ventura, L. et al. Morphological MRI of knee cartilage: repeatability and reproducibility of damage evaluation and correlation with gross pathology examination. Eur Radiol 30, 3226–3235 (2020). https://doi.org/10.1007/s00330-019-06627-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06627-5