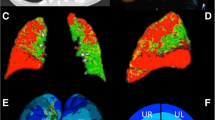
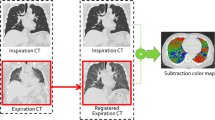
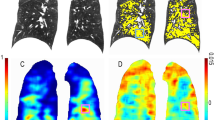
Abstract
Objectives
Chronic obstructive pulmonary disease (COPD) is characterized by variable contributions of emphysema and airway disease on computed tomography (CT), and still little is known on their temporal evolution. We hypothesized that quantitative CT (QCT) is able to detect short-time changes in a cohort of patients with very severe COPD.
Methods
Two paired in- and expiratory CT each from 70 patients with avg. GOLD stage of 3.6 (mean age = 66 ± 7.5, mean FEV1/FVC = 35.28 ± 7.75) were taken 3 months apart and analyzed by fully automatic software computing emphysema (emphysema index (EI), mean lung density (MLD)), air-trapping (ratio expiration to inspiration of mean lung attenuation (E/I MLA), relative volume change between − 856 HU and − 950 HU (RVC856–950)), and parametric response mapping (PRM) parameters for each lobe separately and the whole lung. Airway metrics measured were wall thickness (WT) and lumen area (LA) for each airway generation and the whole lung.
Results
The average of the emphysema parameters (EI, MLD) increased significantly by 1.5% (p < 0.001) for the whole lung, whereas air-trapping parameters (E/I MLA, RVC856–950) were stable. PRMEmph increased from 34.3 to 35.7% (p < 0.001), whereas PRMNormal decrased from 23.6% to 22.8% (p = 0.012). WT decreased significantly from 1.17 ± 0.18 to 1.14 ± 0.19 mm (p = 0.036) and LA increased significantly from 25.08 ± 4.49 to 25.84 ± 4.87 mm2 (p = 0.041) for the whole lung. The generation-based analysis showed heterogeneous results.
Conclusion
QCT detects short-time progression of emphysema in severe COPD. The changes were partly different among lung lobes and airway generations, indicating that QCT is useful to address the heterogeneity of COPD progression.
Key Points
• QCT detects short-time progression of emphysema in severe COPD in a 3-month period.
• QCT is able to quantify even slight parenchymal changes, which were not detected by spirometry.
• QCT is able to address the heterogeneity of COPD, revealing inconsistent changes individual lung lobes and airway generations.



Similar content being viewed by others
Abbreviations
- AS:
-
Active smokers
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- EI:
-
Emphysema index
- E/I MLA:
-
Expiratory to inspiratory ratio of mean lung attenuation
- ES:
-
Ex-smokers
- FEV1:
-
Forced expiratory volume
- GOLD:
-
Global Initiative for Obstructive Lung Disease
- HU:
-
Hounsfield units
- LA:
-
Lumen area
- LLi:
-
Lingula
- LLL:
-
Left lower lobe
- LUL:
-
Left upper lobe
- MEF50 :
-
Maximum expiratory flow after exhalation of 75% of FVC
- MLD:
-
Mean lung density
- PEF:
-
Peak expiratory flow
- PFT:
-
Pulmonary function test
- PRM:
-
Parametric response mapping
- QCT:
-
Quantitative computed tomography
- RML:
-
Middle lobe
- RLL:
-
Right lower lobe
- RUL:
-
Right upper lobe
- RQ:
-
Recent quitters
- RV:
-
Residual volume
- RVC856–950 :
-
Relative volume change between − 856 HU and − 950 HU
- SAD:
-
Small airway disease
- TD:
-
Total diameter
- TLC:
-
Total lung capacity
- TLV:
-
Total lung volume
- VC:
-
Vital capacity
- WP:
-
Wall percentage
- WT:
-
Wall thickness
References
Vogelmeier CF, Criner GJ, Martinez FJ et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 195:557–582
Lynch DA, Al-Qaisi MA (2013) Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging 28:284–290
Coxson HO, Leipsic J, Parraga G, Sin DD (2014) Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med 190:135–144
Labaki WW, Martinez CH, Martinez FJ et al (2017) The role of chest computed tomography in the evaluation and management of the patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 196:1372–1379
Lynch DA, Austin JH, Hogg JC et al (2015) CT-definable subtypes of chronic obstructive pulmonary Disease: A Statement of the Fleischner Society. Radiology. https://doi.org/10.1148/radiol.2015141579:141579
Gevenois PA, De Vuyst P, de Maertelaer V et al (1996) Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 154:187–192
Madani A, Zanen J, Maertelaer V, Gevenois PA (2006) Pulmonary emphysema: objective quantification at multi–detector row CT—comparison with macroscopic and microscopic morphometry. Radiology 238:1036–1043
Hackx M, Bankier AA, Gevenois PA (2012) Chronic obstructive pulmonary disease: CT quantification of airways disease. Radiology 265:34–48
Nakano Y, Wong JC, de Jong PA et al (2005) The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 171:142–146
Galban CJ, Han MK, Boes JL et al (2012) Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 18:1711–1715
Barr RG, Ahmed FS, Carr JJ et al (2012) Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA lung study. Eur Respir J 39:846–854
Gietema HA, Müller NL, Fauerbach PV et al (2011) Quantifying the extent of emphysema:: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 18:661–671
Regan EA, Hokanson JE, Murphy JR et al (2011) Genetic epidemiology of COPD (COPDGene) study design. COPD 7:32–43
Boes JL, Hoff BA, Bule M et al (2015) Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Acad Radiol 22:186–194
Hatt CR, Fernandez-Baldera A, Hoffman EA, Martinez FJ, Galban CJ, Han MK (2017) Reproducibility of parametric response mapping at 30 DAYSC80-C imaging methodology and application to lung disease. (American Thoracic Society international conference abstracts). American Thoracic Society, pp A6502-A6502
Jobst BJ, Weinheimer O, Trauth M et al (2018) Effect of smoking cessation on quantitative computed tomography in smokers at risk in a lung cancer screening population. Eur Radiol 28:807–815
Hasegawa M, Makita H, Nasuhara Y et al (2009) Relationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPD. Thorax 64:332–338
(2019) Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, mangement, and prevention of chronic obstructive pulmonary disease. Available via https://goldcopd.org/wpcontent/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
Quanjer PH, Stanojevic S, Cole TJ et al (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324
Heussel CP, Kappes J, Hantusch R et al (2010) Contrast enhanced CT-scans are not comparable to non-enhanced scans in emphysema quantification. Eur J Radiol 74:473–478
Kauczor HU, Wielputz MO, Owsijewitsch M, Ley-Zaporozhan J (2011) Computed tomographic imaging of the airways in COPD and asthma. J Thorac Imaging 26:290–300
Weinheimer O, Achenbach T, Bletz C, Duber C, Kauczor HU, Heussel CP (2008) About objective 3-d analysis of airway geometry in computerized tomography. IEEE Trans Med Imaging 27:64–74
Wielputz MO, Eichinger M, Weinheimer O et al (2013) Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing. J Thorac Imaging 28:104–113
Wielputz MO, Weinheimer O, Eichinger M et al (2013) Pulmonary emphysema in cystic fibrosis detected by densitometry on chest multidetector computed tomography. PLoS One 8:e73142
Weinheimer O, Wielpütz MO, Konietzke P et al (2017) Fully automated lobe-based airway taper index calculation in a low dose MDCT CF study over 4 time-points. Proc. SPIE 10133, Medical Imaging 2017: Image Processing, 101330U
Konietzke P, Weinheimer O, Wielputz MO et al (2018) Validation of automated lobe segmentation on paired inspiratory-expiratory chest CT in 8-14 year-old children with cystic fibrosis. PLoS One 13:e0194557
Wang Z, Gu S, Leader JK et al (2013) Optimal threshold in CT quantification of emphysema. Eur Radiol 23:975–984
Hersh CP, Washko GR, Estepar RS et al (2013) Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res 14:42
Weinheimer O, Wielpütz MO, Konietzke P et al (2019) Improving pulmonary lobe segmentation on expiratory CTs by using aligned inspiratory CTs SPIE 10950, Medical Imaging 2019: Computer-Aided Diagnosis, 109503I
Weinheimer O, Achenbach T, Düber C (2009) Fully automated extraction of airways from CT scans based on self-adapting region growing. In: Brown M, de Bruijne B, van Ginneken B et al (eds) Proc of second international workshop on pulmonary image analysis (in conjunction with MICCAI) 2009
Grydeland TB, Dirksen A, Coxson HO et al (2009) Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J 34:858–865
Konietzke P, Weinheimer O, Wielpütz MO et al (2018) Quantitative CT detects changes in airway dimensions and air-trapping after bronchial thermoplasty for severe asthma. Eur J Radiol 107:33–38
R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Konietzke P, Jobst B, Wagner WL et al (2018) Similarities in the computed tomography appearance in α1-antitrypsin deficiency and smoking-related chronic obstructive pulmonary disease in a smoking collective. Respiration 96:231–239
Knudsen L, Ochs M (2018) The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 150:661–676
Hogg JC, Macklem PT, Thurlbeck WM (1968) Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 278:1355–1360
Weibel ER (1963) Chapter VI - geometry and dimensions of airways of the respiratory zone. In: Weibel ER (ed) Morphometry of the Human Lung. Academic Press, pp 56–73
Labaki WW, Gu T, Murray S et al (2019) Voxel-wise longitudinal parametric response mapping analysis of chest computed tomography in smokers. Acad Radiol 26:217–223
Diaz AA, Han MK, Come CE et al (2013) Effect of emphysema on CT scan measures of airway dimensions in smokers. Chest 143:687–693
Washko GR, Diaz AA, Kim V et al (2014) Computed tomographic measures of airway morphology in smokers and never-smoking normals. J Appl Physiol (1985) 116:668–673
Zach JA, Newell JD Jr, Schroeder J et al (2012) Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol 47:596–602
Diaz AA, Estépar RSJ, Washko GR (2016) Computed tomographic airway morphology in chronic obstructive pulmonary disease. Remodeling or innate anatomy? Ann Am Thorac Soc 13:4–9
Smith BM, Hoffman EA, Rabinowitz D et al (2014) Comparison of spatially matched airways reveals thinner airway walls in COPD. The multi-ethnic study of atherosclerosis (MESA) COPD study and the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 69:987–996
Cosio M, Ghezzo H, Hogg JC et al (1978) The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 298:1277–1281
Thurlbeck WM, Pun R, Toth J, Frazer RG (1974) Bronchial cartilage in chronic obstructive lung disease. Am Rev Respir Dis 109:73–80
Wielpütz MO, Eberhardt R, Puderbach M, Weinheimer O, Kauczor HU, Heussel CP (2014) Simultaneous assessment of airway instability and respiratory dynamics with low-dose 4D-CT in chronic obstructive pulmonary disease: a technical note. Respiration 87:294–300
Mutuku JK, Chen W-H (2018) Flow characterization in healthy airways and airways with chronic obstructive pulmonary disease (COPD) during different inhalation conditions. Aerosol Air Qual Res 18:2680–2694
Chovancová M, Elcner J (2014) The pressure gradient in the human respiratory tract. EPJ Web Conf 67:02047
Schroeder JD, McKenzie AS, Zach JA et al (2013) Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 201:W460–W470
Nakano Y, Muro S, Sakai H et al (2000) Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162:1102–1108
Hasegawa M, Nasuhara Y, Onodera Y et al (2006) Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173:1309–1315
Hogg JC, Wright JL, Wiggs BR, Coxson HO, Opazo Saez A, Paré PD (1994) Lung structure and function in cigarette smokers. Thorax 49:473–478
Madani A, Van Muylem A, Gevenois PA (2010) Pulmonary emphysema: effect of lung volume on objective quantification at thin-section CT. Radiology 257:260–268
Camiciottoli G, Cavigli E, Grassi L et al (2009) Prevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trial. Eur Radiol 19:58–66
Grydeland TB, Dirksen A, Coxson HO et al (2009) Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J 34:858
Ashraf H, Lo P, Shaker SB et al (2011) Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax 66:55
Funding
This study was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF) to the German Center for Lung Research (DZL) (82DZL004A, 82DZL004A2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Philip Konietzke.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Parts of the lobe segmentation algorithm that are used for labeling of the airways have been licensed to the company Imbio, LCC. There are no further patents, products in development, or marketed products to declare.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 40.4 kb)
Rights and permissions
About this article
Cite this article
Konietzke, P., Wielpütz, M.O., Wagner, W.L. et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol 30, 2502–2512 (2020). https://doi.org/10.1007/s00330-019-06577-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06577-y