Abstract
Objective
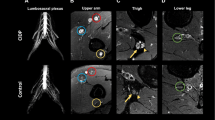
Detection and pattern analysis of fascicular nerve hyperintensities in the T2-weighted image are the backbone of magnetic resonance neurography (MRN) as they may represent lesions of various etiologies. The aim of this study was to assess the prevalence of fascicular nerve hyperintensities in healthy individuals with regard to a potential association with age or cerebral white matter lesions.
Methods
Sixty volunteers without peripheral nerve diseases between the age of 20 and 80 underwent MRN (high-resolution T2-weighted) of upper (median, ulnar, radial) and lower (sciatic, tibial) extremity nerves and a fluid-attenuated inversion recovery (FLAIR) sequence of the brain. Presence of peripheral nerve hyperintensities and degree of cerebral white matter lesions were independently rated by two blinded readers and related to each other and to age. T test with Welch’s correction was used for group comparisons. Spearman’s correlation coefficients were reported for correlation analyses.
Results
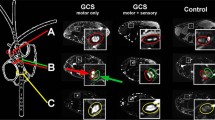
MR neurography revealed fascicular hyperintensities in 10 of 60 subjects (16.7%). Most frequently, they occurred in the sciatic nerve (8/60 subjects, 13.3%), less frequently in the tibial nerve at the lower leg and the median, ulnar, and radial nerves at the upper arm (1.7–5.0%). Mean age of subjects with nerve hyperintensities was higher than that of those without (60.6 years vs. 48.0 years, p = 0.038). There was only a weak correlation of nerve lesions with age and with cerebral white matter lesions, respectively.
Conclusion
Fascicular nerve hyperintensities may occur in healthy individuals and should therefore always be regarded in conjunction with the clinical context.
Key Points
• MR neurography may reveal fascicular hyperintensities in peripheral nerves of healthy individuals. Fascicular hyperintensities occur predominantly in the sciatic nerve and older individuals.
• Therefore, fascicular hyperintensities should only be interpreted as clearly pathologic in conjunction with the clinical context.


Similar content being viewed by others
Abbreviations
- DWMH:
-
Deep white matter hyperintensitites
- FLAIR:
-
Fluid-attenuated inversion recovery
- FOV:
-
Field of view
- MRN:
-
Magnetic resonance neurography
- PVH:
-
Periventricular hyperintensities
- SD:
-
Standard deviation
- T2w:
-
T2-weighted
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- TSE:
-
Turbo spin echo
References
Chhabra A, Andreisek G, Soldatos T et al (2011) MR neurography: past, present, and future. AJR Am J Roentgenol 197:583–591
Kollmer J, Bendszus M, Pham M (2015) MR neurography: diagnostic imaging in the PNS. Clin Neuroradiol 25(Suppl 2):283–289
Kronlage M, Schwehr V, Schwarz D et al (2017) Magnetic resonance neurography: normal values and demographic determinants of nerve caliber and T2 relaxometry in 60 healthy individuals. Clin Neuroradiol. https://doi.org/10.1007/s00062-017-0633-5
Kronlage M, Pitarokoili K, Schwarz D et al (2017) Diffusion tensor imaging in chronic inflammatory demyelinating polyneuropathy: diagnostic accuracy and correlation with electrophysiology. Invest Radiol 52:701–707
Kronlage M, Bäumer P, Pitarokoili K et al (2017) Large coverage MR neurography in CIDP: diagnostic accuracy and electrophysiological correlation. J Neurol 264:1434–1443
Godel T, Bäumer P, Pham M et al (2017) Human dorsal root ganglion in vivo morphometry and perfusion in Fabry painful neuropathy. Neurology 89:1274–1282
Hiltunen J, Kirveskari E, Numminen J, Lindfors N, Göransson H, Hari R (2012) Pre- and post-operative diffusion tensor imaging of the median nerve in carpal tunnel syndrome. Eur Radiol 22:1310–1319
Haakma W, Jongbloed BA, Froeling M et al (2017) MRI shows thickening and altered diffusion in the median and ulnar nerves in multifocal motor neuropathy. Eur Radiol 27:2216–2224
Wu C, Wang G, Zhao Y et al (2017) Assessment of tibial and common peroneal nerves in diabetic peripheral neuropathy by diffusion tensor imaging: a case control study. Eur Radiol 27:3523–3531
Kronlage M, Schwehr V, Schwarz D et al (2018) Peripheral nerve diffusion tensor imaging (DTI): normal values and demographic determinants in a cohort of 60 healthy individuals. Eur Radiol 28:1801–1808
Chhabra A, Belzberg AJ, Rosson GD et al (2016) Impact of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol 26:1235–1244
Pham M, Oikonomou D, Hornung B et al (2015) Magnetic resonance neurography detects diabetic neuropathy early and with proximal predominance. Ann Neurol 78:939–948
Kollmer J, Hund E, Hornung B et al (2015) In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography. Brain 138:549–562
Chhabra A, Carrino JA, Farahani SJ et al (2016) Whole-body MR neurography: prospective feasibility study in polyneuropathy and Charcot-Marie-Tooth disease. J Magn Reson Imaging 44:1513–1521
Upadhyaya V, Upadhyaya DN, Mishra B (2018) MR neurography in traumatic, non-obstetric paediatric brachial plexopathy. Eur Radiol 28:2417–2424
Breitenseher JB, Kranz G, Hold A et al (2015) MR neurography of ulnar nerve entrapment at the cubital tunnel: a diffusion tensor imaging study. Eur Radiol 25:1911–1918
Thawait SK, Chaudhry V, Thawait GK et al (2011) High-resolution MR neurography of diffuse peripheral nerve lesions. AJNR Am J Neuroradiol 32:1365–1372
Pham M, Baumer T, Bendszus M (2014) Peripheral nerves and plexus: imaging by MR-neurography and high-resolution ultrasound. Curr Opin Neurol 27:370–379
Bendszus M, Stoll G (2005) Technology insight: visualizing peripheral nerve injury using MRI. Nat Clin Pract Neurol 1:45–53
Husarik DB, Saupe N, Pfirrmann CW, Jost B, Hodler J, Zanetti M (2009) Elbow nerves: MR findings in 60 asymptomatic subjects--normal anatomy, variants, and pitfalls. Radiology 252:148–156
Hentschel F, Damian M, Krumm B, Froelich L (2007) White matter lesions - age-adjusted values for cognitively healthy and demented subjects. Acta Neurol Scand 115:174–180
Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R (1995) White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 26:1171–1177
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB (2000) Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356:628–634
Fazekas F, Schmidt R, Scheltens P (1998) Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord 9(Suppl 1):2–5
Haller S, Kövari E, Herrmann FR et al (2013) Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic-neuropathologic correlation study. Acta Neuropathol Commun 1:14
de Leeuw FE, de Groot JC, Achten E et al (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70:9–14
Longstreth WT Jr, Manolio TA, Arnold A et al (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 27:1274–1282
Larkman N, Lefebvre G, Jacques T, Demondion X, Cotten H, Cotten A (2017) Anatomical and MR correlative study of the proximal sciatic nerve vasculature. Br J Radiol 90:20170031
Chappell KE, Robson MD, Stonebridge-Foster A et al (2004) Magic angle effects in MR neurography. AJNR Am J Neuroradiol 25:431–440
Chhabra A, Madhuranthakam AJ, Andreisek G (2018) Magnetic resonance neurography: current perspectives and literature review. Eur Radiol 28:698–707
Bendszus M, Rieckmann P, Perez J, Koltzenburg M, Reiners K, Solymosi L (2003) Painful vascular compression syndrome of the sciatic nerve caused by gluteal varicosities. Neurology 61:985–987
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Brinjikji W, Luetmer PH, Comstock B et al (2015) Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 36:811–816
Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341:c3666
Pantoni L, Garcia JH (1995) The significance of cerebral white matter abnormalities 100 years after Binswanger’s report. A review. Stroke 26:1293–1301
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701
Titelbaum DS, Frazier JL, Grossman RI et al (1989) Wallerian degeneration and inflammation in rat peripheral nerve detected by in vivo MR imaging. AJNR Am J Neuroradiol 10:741–746
Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M (2004) MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol 188:171–177
Stanisz GJ, Midha R, Munro CA, Henkelman RM (2001) MR properties of rat sciatic nerve following trauma. Magn Reson Med 45:415–420
Samuelsson K, Press R (2018) Microangiopathy-a potential contributing factor to idiopathic polyneuropathy: a mini review. Front Neurol 9:43
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Moritz Kronlage.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Diffusion tensor imaging, T2-relaxometry and nerve cross sectional area data of the same cohort have been published separately:
-
1.
Kronlage M, Schwehr V, Schwarz D et al (2017) Magnetic Resonance Neurography: Normal Values and Demographic Determinants of Nerve Caliber and T2 Relaxometry in 60 healthy individuals. Clinical Neuroradiology. https://doi.org/10.1007/s00062-017-0633-5
-
2.
Kronlage M, Schwehr V, Schwarz D et al (2018) Peripheral nerve diffusion tensor imaging (DTI): normal values and demographic determinants in a cohort of 60 healthy individuals. European Radiology 28:1801–1808
18 of 60 subjects were used in a control group for:
-
1.
Kronlage M, Bäumer P, Pitarokoili K et al (2017) Large coverage MR neurography in CIDP: diagnostic accuracy and electrophysiological correlation. Journal of Neurology 264:1434–1443
-
2.
Kronlage M, Pitarokoili K, Schwarz D et al (2017) Diffusion Tensor Imaging in Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic Accuracy and Correlation With Electrophysiology. Investigative Radiology 52:701–7075
-
3.
Pitarokoili K, Kronlage M, Bäumer P et al (2018) High-resolution nerve ultrasound and magnetic resonance neurography as complementary neuroimaging tools for chronic inflammatory demyelinating polyneuropathy. Therapeutic Advances in Neurological Disorders 11:1756286418759974
Methodology
• prospective
• cross sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 105 kb)
Rights and permissions
About this article
Cite this article
Kronlage, M., Schwehr, V., Schwarz, D. et al. Prevalence of fascicular hyperintensities in peripheral nerves of healthy individuals with regard to cerebral white matter lesions. Eur Radiol 29, 3480–3487 (2019). https://doi.org/10.1007/s00330-019-06145-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06145-4