Abstract
Objective
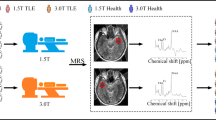
To characterize possible metabolic changes of the dorsolateral prefrontal cortex (DLPFC) in patients with temporal lobe epilepsy (TLE).
Methods
Quantitative proton magnetic resonance spectroscopy (1H-MRS) studies were performed on 24 TLE patients and 22 healthy controls. Metabolite concentrations were calculated using a linear combination model (LCModel) and corrected for cerebrospinal fluid contamination. Comparisons were performed between the TLE patients and the controls and between the left DLPFC and right DLPFC in each group. Pearson correlation coefficients were calculated between the metabolite concentrations and epilepsy duration and between the metabolite concentrations and voxel tissue composition: [gray matter (GM)/(GM+white matter (WM))].
Results
Metabolic asymmetry was found in controls between the left and right DLPFC, i.e., the NAA concentration of the left DLPFC was significantly higher than that of the right. However, such metabolic asymmetry was not observed in TLE patients. Compared with the controls, TLE patients showed significantly decreased NAA and Ins, and the reductions were greater in the left DLPFC. No significant correlation was found between the metabolite concentrations and epilepsy duration or between the metabolite concentrations and voxel tissue composition [GM/(GM+WM)].
Conclusions
This study suggests that TLE can produce metabolic changes to DLPFC that is remote from the seizure focus.
Key Points
• Magnetic resonance spectroscopy probes the brain metabolism noninvasively.
• Dorsolateral prefrontal reductions in NAA (a neuronal marker) and Ins are observed in TLE.
• Temporal lobe epilepsy can result in metabolic changes remote from the seizure focus.





Similar content being viewed by others
Abbreviations
- 1H-MRS:
-
Proton magnetic resonance spectroscopy
- ANOVA:
-
One-way analysis of variance
- ANTs:
-
Advanced normalization tools
- CHESS:
-
Chemical shift selective
- Cho:
-
Choline, including glycerophosphocholine and phosphocholine
- contra:
-
Contralateral to the epileptic focus
- Cr:
-
Creatine + phosphocreatine
- CSF:
-
Cerebrospinal fluid
- DLPFC:
-
Dorsolateral prefrontal cortex
- GM:
-
Gray matter
- Glx:
-
Glutamate + glutamine
- Ins:
-
Myoinositol
- ipsi:
-
Ipsilateral to the epileptic focus
- L+R:
-
Average value of the left and right DLPFCs
- L_sub_TLE:
-
Left TLE subgroup
- L:
-
Left
- LCModel:
-
Linear combination model
- LSD:
-
Least significant difference
- MPRAGE:
-
Magnetization prepared rapid gradient echo
- NAA:
-
N-acetyl aspartate
- PRESS:
-
Point-resolved spectroscopy
- R_sub_TLE:
-
Right TLE subgroup
- R:
-
Right
- TLE:
-
Temporal lobe epilepsy
- VOIs:
-
Volumes of interest
- WM:
-
White matter
References
Sidek S, Ramli N (2016) In vivo proton magnetic resonance spectroscopy (1H- MRS) evaluation of the metabolite concentration of optic radiation in primary open angle glaucoma. Eur Radiol 26(12):4404–4412
Ranjeva JP, Confort-Gouny S, Le Fur Y et al (2000) Magnetic resonance spectroscopy of brain in epilepsy. Childs Nerv Syst 16(4):235–241
Lu JJ, Ren LK, Feng F et al (2006) Metabolic abnormalities in mesial temporal lobe epilepsy patients depicted by proton MR spectroscopy using a 3. 0t MR scanner. Chin Med Sci J 21(4):209–213
Aydin H, Oktay NA, Kizilgoz V, Altin E, Tatar IG, Hekimoglu B (2012) Value of proton-MR-spectroscopy in the diagnosis of temporal lobe epilepsy; correlation of metabolite alterations with electroencephalography. Iran J Radiol 9(1):1–11
Li LM, Dubeau F, Andermann F, Arnold DL (2000) Proton magnetic resonance spectroscopic imaging studies in patients with newly diagnosed partial epilepsy. Epilepsia 41(7):825–831
Krsek P, Hajek M, Dezortova M et al (2007) 1H MR spectroscopic imaging in patients with MRI-negative extratemporal epilepsy: correlation with ictal onset zone and histopathology. Eur Radiol 17(8):2126–2135
Lieb JP, Dasheiff RM, Engel J Jr (1991) Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia 32(6):822–837
Drake M, Allegri RF, Thomson A (2000) Executive cognitive alteration of prefrontal type in patients with mesial temporal lobe epilepsy. Medicina (B Aires) 60(4):453–456
Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P (2007) Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 13(1):12–20
Paik E (1998) Functions of the prefrontal cortex in the human brain. J Korean Med Sci 13(6):569–581
Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14(4):260–264
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30(6):672–679
Hammen T, Hildebrandt M, Stadlbauer A et al (2008) Non-invasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed 21(6):545–552
Yue Q, Liu M, Nie X et al (2012) Quantitative 3.0T MR spectroscopy reveals decreased creatine concentration in the dorsolateral prefrontal cortex of patients with social anxiety disorder. PLoS One 7(10):e48105
Woermann FG, McLean MA, Bartlett PA, Parker GJ, Barker GJ, Duncan JS (1999) Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging-negative temporal lobe epilepsy: different biochemical profile compared with hippocampal sclerosis. Ann Neurol 45(3):369–376
Petroff OA, Errante LD, Kim JH, Spencer DD (2003) N-acetyl-aspartate, total creatine, and myo-inositol in the epileptogenic human hippocampus. Neurology 60(10):1646–1651
Bernard D, Walker PM, Baudouin-Poisson N et al (1996) Asymmetric metabolic profile in mesial temporal lobes: localized H-1 MR spectroscopy in healthy right-handed and non-right-handed subjects. Radiology 199(2):381–389
Riederer F, Bittsansky M, Schmidt C et al (2006) 1H magnetic resonance spectroscopy at 3 T in cryptogenic and mesial temporal lobe epilepsy. NMR Biomed 19(5):544–553
Jayasundar R (2002) Human brain: biochemical lateralization in normal subjects. Neurol India 50(3):267–271
Rademacher J, Burgel U, Geyer S et al (2001) Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain 124(Pt 11):2232–2258
Amunts K, Schlaug G, Schleicher A et al (1996) Asymmetry in the human motor cortex and handedness. Neuroimage 4(3 Pt 1):216–222
Gur RC, Packer IK, Hungerbuhler JP et al (1980) Differences in the distribution of gray and white matter in human cerebral hemispheres. Science 207(4436):1226–1228
Wellard RM, Briellmann RS, Prichard JW, Syngeniotis A, Jackson GD (2003) Myoinositol abnormalities in temporal lobe epilepsy. Epilepsia 44(6):815–821
Capizzano AA, Vermathen P, Laxer KD et al (2002) Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. AJNR Am J Neuroradiol 23(8):1359–1368
Mueller SG, Laxer KD, Cashdollar N, Flenniken DL, Matson GB, Weiner MW (2004) Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia 45(4):355–366
Mueller SG, Ebel A, Barakos J et al (2011) Widespread extrahippocampal NAA/(Cr+Cho) abnormalities in TLE with and without mesial temporal sclerosis. J Neurol 258(4):603–612
Vermathen P, Laxer KD, Schuff N, Matson GB, Weiner MW (2003) Evidence of neuronal injury outside the medial temporal lobe in temporal lobe epilepsy: N-acetylaspartate concentration reductions detected with multisection proton MR spectroscopic imaging--initial experience. Radiology 226(1):195–202
Fisher SK, Novak JE, Agranoff BW (2002) Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 82(4):736–754
Brand A, Leibfritz D, Richter-Landsberg C (1999) Oxidative stress-induced metabolic alterations in rat brain astrocytes studied by multinuclear NMR spectroscopy. J Neurosci Res 58(4):576–585
Flugel D, McLean MA, Simister RJ, Duncan JS (2006) Magnetisation transfer ratio of choline is reduced following epileptic seizures. NMR Biomed 19(2):217–222
Lunsing RJ, Strating K, de Koning TJ, Sijens PE (2017) Diagnostic value of MRS-quantified brain tissue lactate level in identifying children with mitochondrial disorders. Eur Radiol 27(3):976–984
Petroff OA, Pleban LA, Spencer DD (1995) Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn Reson Imaging 13(8):1197–1211
Olsen RW, Avoli M (1997) GABA and epileptogenesis. Epilepsia 38(4):399–407
Sherwin A, Robitaille Y, Quesney F et al (1988) Excitatory amino acids are elevated in human epileptic cerebral cortex. Neurology 38(6):920–923
Westman E, Spenger C, Wahlund LO, Lavebratt C (2007) Carbamazepine treatment recovered low N-acetylaspartate+N-acetylaspartylglutamate (tNAA) levels in the megencephaly mouse BALB/cByJ-Kv1.1(mceph/mceph). Neurobiol Dis 26(1):221–228
Campos BA, Yasuda CL, Castellano G, Bilevicius E, Li LM, Cendes F (2010) Proton MRS may predict AED response in patients with TLE. Epilepsia 51(5):783–788
Acknowledgements
We acknowledge Dr. Richard A.E. Edden for his assistance during the revision of the manuscript.
Funding
This study was funded by National Science Foundation of China (grant nos. 81371528 and 8130118) and the Sichuan Provincial Foundation of Since and Technology (grant no. 2013SZ0047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Yue.
Conflict of interest
The authors of this manuscript declare no relationships with any companies.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all patients in this study.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Tan, Q., Sun, H., Wang, W. et al. Quantitative MR spectroscopy reveals metabolic changes in the dorsolateral prefrontal cortex of patients with temporal lobe epilepsy. Eur Radiol 28, 4496–4503 (2018). https://doi.org/10.1007/s00330-018-5443-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5443-x