Abstract
Objective
To evaluate the magnetic susceptibility, ∆χ v , as a surrogate marker of venous blood oxygen saturation, S v O 2, in second- and third-trimester normal human foetuses.
Methods
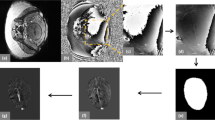
Thirty-six pregnant women, having a mean gestational age (GA) of 31 2/7 weeks, underwent magnetic resonance imaging (MRI). Susceptibility-weighted imaging (SWI) data from the foetal brain were acquired. ∆χ v of the superior sagittal sinus (SSS) was quantified using MR susceptometry from the intra-vascular phase measurements. Assuming the magnetic property of foetal blood, ∆χ do , is the same as that of adult blood, S v O 2 was derived from the measured Δχ v . The variation of ∆χ v and S v O 2, as a function of GA, was statistically evaluated.
Results
The mean ∆χ v in the SSS in the second-trimester (n = 8) and third-trimester foetuses (n = 28) was found to be 0.34± 0.06 ppm and 0.49 ±0.05 ppm, respectively. Correspondingly, the derived S v O 2 values were 69.4% ±3.27% and 62.6% ±3.25%. Although not statistically significant, an increasing trend (p = 0.08) in Δχ v and a decreasing trend (p = 0.22) in S v O 2 with respect to advancing gestation was observed.
Conclusion
We report cerebral venous blood magnetic susceptibility and putative oxygen saturation in healthy human foetuses. Cerebral oxygen saturation in healthy human foetuses, despite a slight decreasing trend, does not change significantly with advancing gestation.
Key points
• Cerebral venous magnetic susceptibility and oxygenation in human foetuses can be quantified.
• Cerebral venous oxygenation was not different between second- and third-trimester foetuses.
• Foetal cerebral venous oxygenation does not change significantly with advancing gestation.



Similar content being viewed by others
Abbreviations
- SWI:
-
Susceptibility-weighted imaging
- MRI:
-
Magnetic resonance imaging
- SSS:
-
Superior sagittal sinus
- S v O 2 :
-
Venous oxygen saturation
- ∆χ v :
-
Magnetic susceptibility
- ∆χ do :
-
Magnetic susceptibility difference between fully oxygenated and deoxygenated foetal blood
- Hct :
-
Haematocrit
- HbF:
-
Foetal haemoglobin
- HbA:
-
Adult haemoglobin
- FHR:
-
Foetal heart rate
- GA:
-
Gestational age
References
Schneider H (2011) Oxygenation of the placental–fetal unit in humans. Respir Physiol Neurobiol 178:51–58
Baschat AA (2004) Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet Gynecol Surv 59:617–627
Baschat DAA (2004) Fetal responses to placental insufficiency: an update. BJOG Int J Obstet Gynaecol 111:1031–1041
Valcamonico A et al (1994) Absent end-diastolic velocity in umbilical artery: risk of neonatal morbidity and brain damage. Am J Obstet Gynecol 170:796–801
Gramellini D et al (1992) Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol 79:416–420
Hecher K et al (1992) Potential for diagnosing imminent risk to appropriate-and small-for-gestational-age fetuses by Doppler sonographic examination of umbilical and cerebral arterial blood flow. Ultrasound Obstet Gynecol 2:266–271
Al-Ghazali W et al (1989) Evidence of redistribution of cardiac output in asymmetrical growth retardation. BJOG Int J Obstet Gynaecol 96:697–704
Ferrazzi E et al (2002) Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol 19:140–146
Dubiel M, Gunnarsson G, Gudmundsson S (2002) Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet Gynecol 20:117–121
Wladimiroff J, Tonge H, Stewart P (1986) Doppler ultrasound assessment of cerebral blood flow in the human fetus. BJOG Int J Obstet Gynaecol 93:471–475
Rosen K, Kjellmer I (1975) Changes in the fetal heart rate and ECG during hypoxia. Acta Physiol 93:59–66
Parer J et al (1980) Increased fetal heart rate variability with acute hypoxia in chronically instrumented sheep. Eur J Obstet Gynecol Reprod Biol 10:393–399
Smith J et al (1988) Antenatal fetal heart rate variation in relation to the respiratory and metabolic status of the compromised human fetus. BJOG Int J Obstet Gynaecol 95:980–989
Gagnon R, Johnston L, Murotsuki J (1996) Fetal placental embolization in the late-gestation ovine fetus: alterations in umbilical blood flow and fetal heart rate patterns. Am J Obstet Gynecol 175:63–72
Ribbert LS et al (1991) Relation of fetal blood gases and data from computer-assisted analysis of fetal heart rate patterns in small for gestation fetuses. BJOG Int J Obstet Gynaecol 98:820–823
Chipchase J et al (2002) Cerebral hemoglobin concentration and oxygen saturation measured by intensity modulated optical spectroscopy in the human fetus during labor. J Perinat Med 30:502–509
Vintzileos AM et al (2005) Transabdominal fetal pulse oximetry with near-infrared spectroscopy. Am J Obstet Gynecol 192:129–133
Rutherford M et al (2008) MR imaging methods for assessing fetal brain development. Develop Neurobiol 68:700–711
Levine D et al (2003) Fast MR imaging of fetal central nervous system abnormalities 1. Radiology 229:51–61
Whitby E et al (2004) Comparison of ultrasound and magnetic resonance imaging in 100 singleton pregnancies with suspected brain abnormalities. BJOG Int J Obstet Gynaecol 111:784–792
Wolfberg AJ et al (2007) Identification of fetal cerebral lactate using magnetic resonance spectroscopy. Am J Obstet Gynecol 196:e9–e11
Cetin I et al (2011) Lactate detection in the brain of growth-restricted fetuses with magnetic resonance spectroscopy. Am J Obstet Gynecol 205:350. e1–350. e7
Zhu MY et al (2016) The hemodynamics of late-onset intrauterine growth restriction by MRI. Am J Obstet Gynecol 214:367. e1–367. e17
Stuber M et al (2002) Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med 48:425–429
Frayne R et al (2003) Magnetic resonance imaging at 3.0 Tesla: challenges and advantages in clinical neurological imaging. Investig Radiol 38:385–402
Haacke EM et al (1997) In vivo measurement of blood oxygen saturation using magnetic resonance imaging: A direct validation of the blood oxygen level-dependent concept in functional brain imaging. Hum Brain Mapp 5:341–346
Fernández-Seara MA et al (2006) MR susceptometry for measuring global brain oxygen extraction. Magn Reson Med 55:967–973
Vegh V et al. (2015) Selective channel combination of MRI signal phase. Magnetic Resonance in Medicine
Neelavalli J et al (2014) Measuring venous blood oxygenation in fetal brain using susceptibility-weighted imaging. J Magn Reson Imaging 39:998–1006
Cheema R et al (2004) Fetal cerebral venous Doppler velocimetry in normal and high-risk pregnancy. Ultrasound Obstet Gynecol 24:147–153
Hadlock FP et al (1985) Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol 151:333–337
Wang Y et al (2000) Artery and vein separation using susceptibility-dependent phase in contrast-enhanced MRA. J Magn Reson Imaging 12:661–670
Boulot P et al (1993) Hematologic values of fetal blood obtained by means of cordocentesis. Fetal Diagn Ther 8:309–316
Jain V, Langham MC, Wehrli FW (2010) MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 30:1598–1607
Spees WM et al (2001) Water proton MR properties of human blood at 1.5 Tesla: Magnetic susceptibility, T1, T2, T* 2, and non-Lorentzian signal behavior. Magn Reson Med 45:533–542
Schroter B et al (1997) Normal value curves for intrauterine fetal blood gas and acid-base parameters in the 2nd and 3rd trimester. Gynakol Geburtshilfliche Rundsch 37:130–135 Article in German
Jain V et al (2014) Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab 34:380–388
Burton G, Jaunaiux E (2001) Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynécologie Obstétrique Fertilité 29:503–508
Veille J-C, Hanson R, Tatum K (1993) Longitudinal quantitation of middle cerebral artery blood flow in normal human fetuses. Am J Obstet Gynecol 169:1393–1398
Nield LE et al (2002) MRI-based blood oxygen saturation measurements in infants and children with congenital heart disease. Pediatr Radiol 32:518–522
Liu P et al (2014) Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO2) in neonates using MRI. NMR Biomed 27:332–340
Chua S et al (1997) Fetal oxygen saturation during labour. BJOG Int J Obstet Gynaecol 104:1080–1083
Dildy GA, Loucks CA, Clark SL (1993) Intrapartum fetal pulse oximetry in the presence of fetal cardiac arrhythmia. Am J Obstet Gynecol 169:1609–1611
Dildy GA et al (1996) The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol 175:682–687
Avni R et al (2016) MR Imaging-derived oxygen-hemoglobin dissociation curves and fetal-placental oxygen-hemoglobin affinities. Radiology 280:68–77
Bard H et al (1970) The relative rates of synthesis of hemoglobins A and F in immature red cells of newborn infants. Pediatrics 45:766–772
Weisskoff RM, Kiihne S (1992) MRI susceptometry: Image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med 24:375–383
Liu C et al (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41
Johnson G, Wadghiri YZ, Turnbull DH (1999) 2 D multislice and 3 D MRI sequences are often equally sensitive. Magn Reson Med 41:824–828
Krishnamurthy U et al (2015) MR imaging of the fetal brain at 1.5 T and 3.0 T field strengths: comparing specific absorption rate (SAR) and image quality. J Perinat Med 43:209–220
Acknowledgements
The authors would like to thank Maria Cabrera and the research staff at the PRB for their help in volunteer recruitment.
Funding
This research was supported, in part, by the Perinatology Research Branch (PRB), Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); in part, with Federal funds from NICHD/NIH/DHHS under contract no. HHSN275201300006C; and an STTR grant from the NHLBI no. 1R42HL112580- 01A1.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Jaladhar Neelavalli, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Yadav, B.K., Krishnamurthy, U., Buch, S. et al. Imaging putative foetal cerebral blood oxygenation using susceptibility weighted imaging (SWI). Eur Radiol 28, 1884–1890 (2018). https://doi.org/10.1007/s00330-017-5160-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5160-x