Abstract
Purpose
To compare inter-reader concordance and accuracy of qualitative diffusion-weighted (DW) PIRADSv2.0 score with those of quantitative DW-MRI for the diagnosis of peripheral zone prostate cancer.
Materials and methods
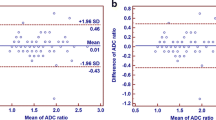
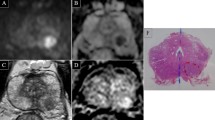
Two radiologists independently assigned a DW-MRI-PIRADS score to 92 PZ-foci, in 74 patients (64.3±5.6 years old; median PSA level: 8 ng/ml, normal DRE in 70 men). A standardised ADCmean and nine ADC-derived parameters were measured, including ADCratios with the whole-prostate (WP-ADCratio) or the mirror-PZ (mirror-ADCratio) as reference areas. Surgical histology and MRI-TRUS fusion-biopsy were the reference for tumours and benign foci, respectively. Inter-reader agreement was assessed by the Cohen-kappa-coefficient and the intraclass correlation coefficient (ICC). Univariate-multivariate regressions determined the most predictive factor for cancer.
Results
Fifty lesions were malignant. Inter-reader concordance was fair for qualitative assessment, but excellent for quantitative assessment for all quantitative variables. At univariate analysis, ADCmean, WP-ADCratio and WL-ADCmean performed equally, but significantly better than the mirror-ADCratio (p<0.001). At multivariate analysis, the only independent variable significantly associated with malignancy was the whole-prostate-ADCratio. At a cut-off value of 0.68, sensitivity was 94–90 % and specificity was 60–38 % for readers 1 and 2, respectively.
Conclusion
The whole-prostate-ADCratio improved the qualitative inter-reader concordance and characterisation of focal PZ-lesions.
Key Points
• Inter-reader concordance of DW PI-RADSv2.0 score for PZ lesions was only fair.
• Using a standardised ADCmean measurement and derived DW-quantitative parameters, concordance was excellent.
• The whole-prostate ADCratio performed significantly better than the mirror-ADCratio for cancer detection.
• At a cut-off of 0.68, sensitivity values of WP-ADCratio were 94–90 %.
• The whole-prostate ADCratio may circumvent variations of ADC metrics across centres.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DW:
-
Diffusion-weighted
- EPI:
-
Echo planar imaging
- ICC:
-
Intraclass correlation coefficient
- mpMRI:
-
Multiparametric MRI
- OR:
-
Odds ratio
- PZ:
-
Peripheral zone
- ROI:
-
Region of interest
- SI:
-
Signal intensity
- TZ:
-
Transition zone
- WP:
-
Whole prostate
References
Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW et al (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261:46–66
Baur AD, Maxeiner A, Franiel T, Kilic E, Huppertz A, Schwenke C et al (2014) Evaluation of the prostate imaging reporting and data system for the detection of prostate cancer by the results of targeted biopsy of the prostate. Investig Radiol 49:411–420
Vargas HA, Hotker AM, Goldman DA, Moskowitz CS, Gondo T, Matsumoto K et al (2016) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 26:1606–1612
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ et al (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69:16–40
Rosenkrantz AB, Oto A, Turkbey B, Westphalen AC (2016) Prostate Imaging Reporting and Data System (PI-RADS), Version 2: A Critical Look. AJR Am J Roentgenol 206:1179–1183
Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B et al (2016) Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 280:793–780
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822
Mertan FV, Greer MD, Shih JH, George AK, Kongnyuy M, Muthigi A et al (2016) Prospective Evaluation of the Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection. J Urol 196:690–696
Syed JS, Nguyen KA, Nawaf CB, Bhagat AM, Huber S, Levi A et al (2017) Prostate zonal anatomy correlates with the detection of prostate cancer on multiparametric magnetic resonance imaging/ultrasound fusion-targeted biopsy in patients with a solitary PI-RADS v2-scored lesion. Urol Oncol. https://doi.org/10.1016/j.urolonc.2017.04.011
Nagel KN, Schouten MG, Hambrock T, Litjens GJ, Hoeks CM, ten Haken B et al (2013) Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology 267:164–172
Rosenkrantz AB, Khalef V, Xu W, Babb JS, Taneja SS, Doshi AM (2015) Does normalisation improve the diagnostic performance of apparent diffusion coefficient values for prostate cancer assessment? A blinded independent-observer evaluation. Clin Radiol 70:1032–1037
De Cobelli F, Ravelli S, Esposito A, Giganti F, Gallina A, Montorsi F et al (2015) Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: comparison with prostate biopsy and radical prostatectomy specimen. AJR Am J Roentgenol 204:550–557
Barrett T, Priest AN, Lawrence EM, Goldman DA, Warren AY, Gnanapragasam VJ et al (2015) Ratio of Tumor to Normal Prostate Tissue Apparent Diffusion Coefficient as a Method for Quantifying DWI of the Prostate. AJR Am J Roentgenol 205:W585–W593
Lebovici A, Sfrangeu SA, Feier D, Caraiani C, Lucan C, Suciu M et al (2014) Evaluation of the normal-to-diseased apparent diffusion coefficient ratio as an indicator of prostate cancer aggressiveness. BMC Med Imaging 14:15
Rosenkrantz AB, Meng X, Ream JM, Babb JS, Deng FM, Rusinek H et al (2016) Likert score 3 prostate lesions: Association between whole-lesion ADC metrics and pathologic findings at MRI/ultrasound fusion targeted biopsy. J Magn Reson Imaging 43:325–332
Vural M, Ertas G, Onay A, Acar O, Esen T, Saglican Y et al Conspicuity of Peripheral Zone Prostate Cancer on Computed Diffusion-Weighted Imaging: Comparison of cDWI1500, cDWI2000, and cDWI3000. Biomed Res Int. https://doi.org/10.1155/2014/768291
Feuerlein S, Davenport MS, Krishnaraj A, Merkle EM, Gupta RT (2015) Computed high b-value diffusion-weighted imaging improves lesion contrast and conspicuity in prostate cancer. Prostate Cancer Prostatic Dis 18:155–160
Zhang K, Shen Y, Zhang X, Ma L, Wang H, An N et al (2016) Predicting Prostate Biopsy Outcomes: A Preliminary Investigation on Screening with Ultrahigh B-Value Diffusion-Weighted Imaging as an Innovative Diagnostic Biomarker. PLoS One 11:e0151176. https://doi.org/10.1371/journal.pone.0151176
Boesen L, Chabanova E, Logager V, Balslev I, Thomsen HS (2015) Apparent diffusion coefficient ratio correlates significantly with prostate cancer gleason score at final pathology. J Magn Reson Imaging 42:446–453
Donati OF, Afaq A, Vargas HA, Mazaheri Y, Zheng J, Moskowitz CS et al (2014) Prostate MRI: Evaluating Tumor Volume and Apparent Diffusion Coefficient as Surrogate Biomarkers for Predicting Tumor Gleason Score. Clin Cancer Res 20:3705–3711
Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA et al (2011) International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 1: specimen handling. Mod Pathol 24:6–15
Epstein JI (2010) An update of the Gleason grading system. J Urol 183:433–440
Delongchamps NB, Lefevre A, Bouazza N, Beuvon F, Legman P, Cornud F (2015) Detection of significant prostate cancer with magnetic resonance targeted biopsies--should transrectal ultrasound-magnetic resonance imaging fusion guided biopsies alone be a standard of care? J Urol 193:1198–1204
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Bratan F, Melodelima C, Souchon R, Hoang Dinh A, Mege-Lechevallier F, Crouzet S et al (2015) How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 275:144–154
Cornud F, Khoury G, Bouazza N, Beuvon F, Peyromaure M, Flam T et al (2014) Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol 191:1272–1279
Muller BG, Shih JH, Sankineni S, Marko J, Rais-Bahrami S, George A et al (2015) Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology 277:741–750
Maas MC, Futterer JJ, Scheenen TW (2013) Quantitative evaluation of computed high B value diffusion-weighted magnetic resonance imaging of the prostate. Investig Radiol 48:779–786
Metens T, Miranda D, Absil J, Matos C (2012) What is the optimal b value in diffusion-weighted MR imaging to depict prostate cancer at 3T? Eur Radiol 22:703–709
Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C et al (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259:775–784
Kitajima K, Takahashi S, Ueno Y, Yoshikawa T, Ohno Y, Obara M et al (2012) Clinical utility of apparent diffusion coefficient values obtained using high b-value when diagnosing prostate cancer using 3 tesla MRI: comparison between ultra-high b-value (2000 s/mm(2)) and standard high b-value (1000 s/mm(2)). J Magn Reson Imaging 36:198–205
Thormer G, Otto J, Reiss-Zimmermann M, Seiwerts M, Moche M, Garnov N et al (2012) Diagnostic value of ADC in patients with prostate cancer: influence of the choice of b values. Eur Radiol 22:1820–1828
Scheenen TW, Rosenkrantz AB, Haider MA, Futterer JJ (2015) Multiparametric Magnetic Resonance Imaging in Prostate Cancer Management: Current Status and Future Perspectives. Investig Radiol 50:594–600
Ukimura O, Desai MM, Palmer S, Valencerina S, Gross M, Abreu AL et al (2012) 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol 187:1080–1086
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is François Cornud.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise: frantz.foissac@aphp.fr
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was waived by the ethics committee.
Methodology
• retrospective
• case-control study
• multicentre study
Rights and permissions
About this article
Cite this article
Pierre, T., Cornud, F., Colléter, L. et al. Diffusion-weighted imaging of the prostate: should we use quantitative metrics to better characterize focal lesions originating in the peripheral zone?. Eur Radiol 28, 2236–2245 (2018). https://doi.org/10.1007/s00330-017-5107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5107-2