Abstract
Objectives
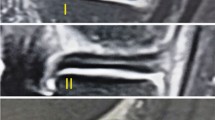
To investigate the association of weight change over 48 months with progression of meniscal intrasubstance degeneration (MID).
Methods
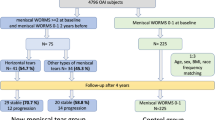
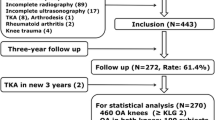
We studied 487 subjects with MID at baseline and after 48 months using 3-T MRI with the same protocol (FSE sequences with and without fat suppression). These participants lost weight (≥3%, n = 141), had moderate weight gain (3–10%, n = 77), substantial weight gain (>10%, n = 15) or maintained stable weight (n = 254). Progression of MID to a meniscal tear was assessed using the WORMS grading system and compared among weight change groups using logistic regression. ANOVA and chi-square tests were used to study the differences in subjects’ characteristics.
Results
Progression of MID increased from weight loss to substantial weight gain (p < 0.001) and was significantly more likely with both moderate weight gain (odds ratio [OR], 4.9; 95% confidence interval [CI] 2.4–8.9) and substantial weight gain (OR, 9.5; 95% CI 3.2–28.5) compared to stable weight. Results were similar in both menisci for moderate weight gain (medial: OR, 6.8; 95% CI 3.5–11.3; lateral: OR, 2.6; 95% CI 1.1–6.6) and substantial weight gain (medial: OR, 21.0; 95% CI 5.1–80.7; lateral: OR, 9.7; 95% CI 0.95–100.2).
Conclusion
Weight gain is associated with an increased likelihood that meniscal intrasubstance degeneration will progress with the risk increasing with greater weight gain.
Key Points
• Subjects who gained weight were more likely to develop meniscal tears.
• Greater amount of weight gain was associated with an increasing likelihood of progression.
• Prevention of weight gain has health benefits for the meniscus.




Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- HKA:
-
Angle hip-knee-ankle angle
- KL:
-
Kellgren–Lawrence
- MID:
-
Meniscal intrasubstance degeneration
- MRI:
-
Magnetic resonance imaging
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis Initiative
- RMSE:
-
Root mean square error
- WORMS:
-
Whole-Organ Magnetic Resonance Imaging Score
References
Fithian DC, Kelly MA, Mow VC (1990) Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res 252:19–31
Englund M, Guermazi A, Lohmander SL (2009) The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am 47:703–712
Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE et al (2009) Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 60:831–839
Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC et al (2005) Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology 237:998–1007
Englund M, Roos EM, Lohmander LS (2003) Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum 48:2178–2187
Oei EH, Nikken JJ, Verstijnen AC, Ginai AZ, Myriam Hunink MG (2003) MR imaging of the menisci and cruciate ligaments: a systematic review. Radiology 226:837–848
Kumm J, Roemer FW, Guermazi A, Turkiewicz A, Englund M (2016) Natural history of intrameniscal signal intensity on knee MR images: six years of data from the Osteoarthritis Initiative. Radiology 278:164–171
Crema MD, Hunter DJ, Roemer FW, Li L, Marra MD, Nogueira-Barbosa MH et al (2011) The relationship between prevalent medial meniscal intrasubstance signal changes and incident medial meniscal tears in women over a 1-year period assessed with 3.0 T MRI. Skelet Radiol 40:1017–1023
De Smet AA, Tuite MJ (2006) Use of the "two-slice-touch" rule for the MRI diagnosis of meniscal tears. AJR Am J Roentgenol 187:911–914
Stoller DW, Martin C, Crues JV 3rd, Kaplan L, Mink JH (1987) Meniscal tears: pathologic correlation with MR imaging. Radiology 163:731–735
Hajek PC, Gylys-Morin VM, Baker LL, Sartoris DJ, Haghighi P, Resnick D (1987) The high signal intensity meniscus of the knee. Magnetic resonance evaluation and in vivo correlation. Invest Radiol 22:883–890
Low AK, Chia MR, Carmody DJ, Lucas P, Hale D (2008) Clinical significance of intrasubstance meniscal lesions on MRI. J Med Imaging Radiat Oncol 52:227–230
Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC et al (2015) Association of cartilage degeneration with four year weight gain–3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 23:525–531
Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G et al (2016) Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 24:1126–1134
Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC et al (2015) Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J Magn Reson Imaging 41:1272–1280
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM (2010) Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 18:776–786
Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C et al (2012) Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. J Magn Reson Imaging 35:370–378
Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE et al (2011) Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage 19:65–73
Jungmann PM, Kraus MS, Alizai H, Nardo L, Baum T, Nevitt MC et al (2013) Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 65:1942–1950
Cooke TD, Li J, Scudamore RA (1994) Radiographic assessment of bony contributions to knee deformity. Orthop Clin North Am 25:387–393
Yoshioka Y, Siu D, Cooke TD (1987) The anatomy and functional axes of the femur. J Bone Joint Surg Am 69:873–880
Yoshioka Y, Siu DW, Scudamore RA, Cooke TD (1989) Tibial anatomy and functional axes. J Orthop Res 7:132–137
Hovis KK, Alizai H, Tham SC, Souza RB, Nevitt MC, McCulloch CE et al (2012) Non-traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the Osteoarthritis Initiative (OAI). Skeletal Radiol 41:1435–1443
Neame R, Zhang W, Deighton C, Doherty M, Doherty S, Lanyon P et al (2004) Distribution of radiographic osteoarthritis between the right and left hands, hips, and knees. Arthritis Rheum 50:1487–1494
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D et al (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190
Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J et al (2012) Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res 64:248–255
Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC et al (2011) Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology 261:507–515
De Smet AA, Graf BK (1994) Meniscal tears missed on MR imaging: relationship to meniscal tear patterns and anterior cruciate ligament tears. AJR Am J Roentgenol 162:905–911
Hart DJ, Spector TD (1993) The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol 20:331–335
De Smet AA, Norris MA, Yandow DR, Quintana FA, Graf BK, Keene JS (1993) MR diagnosis of meniscal tears of the knee: importance of high signal in the meniscus that extends to the surface. AJR Am J Roentgenol 161:101–107
Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF (1988) Obesity and knee osteoarthritis. The Framingham Study. Ann Internal Med 109:18–24
Christensen R, Bartels EM, Astrup A, Bliddal H (2007) Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 66:433–439
Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ (1992) Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med 116:535–539
Manninen P, Riihimaki H, Heliovaara M, Suomalainen O (2004) Weight changes and the risk of knee osteoarthritis requiring arthroplasty. Ann Rheum Dis 63:1434–1437
Teichtahl AJ, Wluka AE, Wang Y, Strauss BJ, Proietto J, Dixon JB et al (2014) The longitudinal relationship between changes in body weight and changes in medial tibial cartilage, and pain among community-based adults with and without meniscal tears. Ann Rheum Dis 73:1652–1658
Wing RR, Phelan S (2005) Long-term weight loss maintenance. Am J Clin nutrition 82:222s-5s
Wluka AE, Lombard CB, Cicuttini FM (2013) Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol 9:225–235
Lombard CB, Deeks AA, Teede HJ (2009) A systematic review of interventions aimed at the prevention of weight gain in adults. Public Health Nutr 12:2236–2246
Costa CR, Morrison WB, Carrino JA (2004) Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol 183:17–23
Vedi V, Williams A, Tennant SJ, Spouse E, Hunt DM, Gedroyc WM (1999) Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg Br 81:37–41
Guimaraes JB, Facchetti L, Schwaiger BJ, Gersing AS, Majumdar S, Ma BC et al (2017) Evolution of intrameniscal signal-intensity alterations detected on MRI over 24 months in patients with traumatic anterior cruciate ligament tear. AJR Am J Roent 208:386–392
Allen DM, Li L, Crema MD, Marra MD, Guermazi A, Wyman BT et al (2010) The relationship between meniscal tears and meniscal position. Ther Adv Musculoskelet Dis 2:315–323
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Thomas Link.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Statistics and biometry
Charles E. McCulloch, Ph.D. kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• multicentre study
Rights and permissions
About this article
Cite this article
Guimaraes, J.B., Nevitt, M.C., McCulloch, C.E. et al. Association of weight change with progression of meniscal intrasubstance degeneration over 48 months: Data from the Osteoarthritis Initiative. Eur Radiol 28, 953–962 (2018). https://doi.org/10.1007/s00330-017-5054-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5054-y